In the previous post, we discussed racemic mixtures which consist of equal amounts of two enantiomers.

Racemic mixtures are not chiral as they are not optically active. For example, 2-bromobutane exists in two stereoisomers, more specifically two enantiomers, R and S enantiomers.

The (R)-2-bromobutane is an optically active compound with a specific rotation of -23.1o. The S enantiomer is also a chiral compound with a specific rotation of +23.1o. However, if the sample is a 1:1 mixture of these enantiomers, then it will not rotate the plane of a polarized light.
This is because each enantiomer rotates the plane of the light to the same extent but opposite directions. The net rotation is simply canceled out and the net rotation is zero.
So, the values for specific rotation of R or S 2-bromobutane are referred to the sample that contains 100% of each enantiomer respectively. These are called enantiomerically/optically pure samples.
Now, remember that the 100% is used to specify that the sample does not contain any of the other enantiomer. It is not to say that there is absolutely nothing else in the sample. Most of the time there is also a solvent whether we are running a reaction or analyzing the sample. In fact, remember that the specific rotation is measured in a 1 dm sample tube at 25 °C using a sodium lamp which emits light at a fixed wavelength of 589 nm. This is called the D line of sodium. The concentration of the chiral solute is kept at 1 g/mL.
Enantiomeric Excess
So far, we have discussed two scenarios when dealing with chiral compound and optical rotation. One was the enantiomerically pure sample and the other was the racemic mixture.
Let’s now consider a mixture that contains, for example, 80% (R)-2-bromobutane and 20% (S)-2-bromobutane. The sample is neither optically pure, nor is it a racemic mixture. There is more of one enantiomer that the other, or, in other words one enantiomer (in this case the (R)-2-bromobutane) is said to be in excess.
In order to quantify this excess, the term enantiomeric excess (ee) is used. Enantiomeric excess tells us how much more of one enantiomer is present in the mixture. In this example, the ee is determined by the difference of percentages of the two enantiomers:
% ee (R) = enantiomer R – enantiomer S = 80% – 20% = 60%
We can visualize this by looking at the boxes representing the mixture of the enantiomers.

Figure A represents the percentage of each enantiomer. There is 20% of the S and 80% of the R enantiomer in the mixture. However, the ee of the R enantiomer is not 80% – it is only 60% as the other 20% of the R enantiomer makes up the quasi-racemic mixture with the 20% of the S enantiomer (Figure B). Figure C shows this part of the mixture as one in green and what is left in the red part is the enantiomeric excess of the R enantiomer.
The composition of a chiral sample may also be given in moles, grams and other units. Let’s consider the following problems:
For example, what is the enantiomeric excess of the mixture containing 12.8 mol (R)-2-bromobutane and 3.2 mol (S)-2-bromobutane? In this case, we should simply find the difference in the moles:
ee = moles (R) – moles (S) = 12.8 – 3.2 = 9.6 mol
There is a 9.6 mole excess of the R enantiomer.
However, the ee is most often expressed in percentage and the percent ee can be calculated by dividing the excess (9.6 mol) of the R enantiomer by the total number of moles for both enantiomers:

You can use the following general formula to calculate the % ee of a sample when the composition is given in any unit other than the percentage:

Enantiomeric Excess and Optical Activity
We learned that racemic mixtures are optically inactive and therefore achiral, and that the specific rotation is given for each pure enantiomer.
So, what is the correlation between the optical rotation and a sample with an enantiomeric excess?
We can determine the amount of each enantiomer in a mixture by measuring its optical rotation.
However, first, let’s emphasize some important features of optical rotation that a sample with enantiomeric excess inherits for the given enantiomer. One is that the direction of the rotation is going to be the same as that of the enantiomer in excess.
For example, if the mixture contains 60% of the R enantiomer and 40% of the S enantiomer, the degree and direction will be based on the specific rotation of the R enantiomer. If it is (+), then the rotation is going to be clockwise and if it is an R-(-) enantiomer, then the rotation will be counterclockwise.
Specific Rotation and Observed Specific Rotation
If we know the amounts of the enantiomers and their specific values are given in literature, the degree of the rotation is calculated by using the excess amount of the enantiomer. For example, if the specific rotation of the R enantiomer is +8.40o, then the mixture containing 60% R will have a specific rotation of +8.40o x 0.20 = +1.68o.
The 0.20 is the enantiomeric excess (60-40=20%). This means that the specific rotation of this sample is 20% of the specific rotation of the R enantiomer.
If we do not know the quantities of the enantiomers in the mixture, we can calculate them by first determining the observed rotation of the sample and turning that into what is called the observed specific rotation of the sample. This number then is used in the following formula linking the % enantiomeric excess, the observed specific rotation, and the specific rotation of the pure enantiomer that is in excess:

I know that these terms, and especially the observed specific rotation can be confusing, but let’s work out an example to make them a bit clearer.
We know what the specific rotation is – it is physical property of a chiral compound determined under specific conditions to keep the data communicable.
The observed specific rotation is determined by us and is only for our experimental purposes, unlike the specific rotation which is a constant literature value.
For example, let’s say we synthesized (S)-2-bromobutane and measured the optical rotation of the sample in a 10.0 cm tube and it appears to be +2.70o. We cannot compare this number to the specific rotation of pure (S)-2-bromobutane (+23.1o) if the conditions for this measurement are not the same as what is set for determining the specific rotation.
Let’s assume that we had 1.46 g of (S)-2-bromobutane dissolved in 10.0 ml of water. Before using these numbers in the formula for finding the specific rotation, we need to make sure the units are correct. The concentration (c) must be in grams per milliliter and therefore, it is 1.46/10 = 0.146 g/mL. The pathlength (l) must be in decimeters (1 dm = 10 cm) and since it is stated as 10.0 cm in the problem, we will use 1.00 dm. Now, we can plug these values into the equation and determine the observed specific rotation:

The observed specific rotation in this case is +18.5o.
So, what does this number indicate considering that the specific rotation is +23.1o?
The fact that we get a different value compared to the literature standard indicates that our synthesis did not quite go as planned and a mixture containing significant amount of the R enantiomer is also obtained.
The good news, however, is that the sample had a positive (dextrorotatory) optical rotation, and this is very important! If it had a counterclockwise rotation, that would mean that the R enantiomer was prepared in excess instead of the S which is not what we planned.
When you are solving a problem, remember that you identify which enantiomer is in excess based on the sings of the observed and specific rotations. Therefore, pay attention to both to the degree (number) and the sign of the rotation.
As an example, let’s say the above example consisted in two questions. One was finding the observed specific rotation and the second is to calculate the enantiomeric excess.
Again, we know that the S enantiomer is in excess because the specific rotation of this isomer (enantiomer) is +23.1 and the sample has a positive optical rotation.

To find the enantiomeric excess, we can now plug in the numbers and calculate the ee of the sample using the formula mentioned earlier:

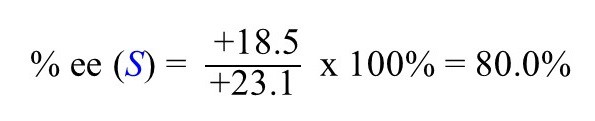
Percentage of Enantiomers from Optical Rotation
Let’s continue further on the example we were working on and also determine the percentage of each enantiomer. We know that the enantiomeric excess of the S enantiomer is 80%, so how many percent of each enantiomer is present in the mixture?
80% excess of the S enantiomer means that the other 20% is a racemic mixture of both S and R. Remember that racemic mixtures have a 1:1 ratio of the enantiomers, so within those 20%, there is 10% of R and additional 10% of the S enantiomer. Thus, the total percentage of the S enantiomer is 80% + 10% = 90% and the R-enantiomer makes the 10% of the entire mixture.
% (S) = 90%, % (R) = 10%
The second way of determining the percentage of each enantiomer from the enantiomeric excess is to set up two equations;
The first equation simply states that the sum of the two enantiomers is 100%:
S + R = 100%
The second equation is based on the definition of the enantiomeric excess. We now know that ee tells us how much more of one enantiomer is present in the mixture compared to the second enantiomer. In other words, it is the difference of the two enantiomers, so for the mixture with an ee of 80%, we can write that
S – R = 80%
By adding these two equations, we get that
S + R = 100%
+
S – R = 80%
2S = 180%. consequently S = 90% and R = 10%

Practice
What is the enantiomeric excess of an adrenaline sample that has a specific rotation of -47.7? Pure adrenaline has a specific rotation of -53.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
How many percent of cholesterol and its enantiomer are present in a sample with an observed specific rotation of -22.4°? The specific rotation of pure cholesterol is -32°.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
The specific rotation of a pure substance is +78°. What is the specific rotation of a mixture containing 75% of this isomer and 25% of the (-) isomer?
a) +78°
b) +39°
c) +59°
d) +20°
e) 0°
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
(+)-Cavicularin has a specific rotation of +168.2°. What would be the specific rotation of a solution which contains 60.0% (-)-Cavicularin and 40.0% (+)-Cavicularin?
a) +33.6°
b) +101°
c) -33.6°
d) -101°
e) -67.3°
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
The specific rotation of (S)-alanine is +8.5°. If a mixture of alanine enantiomers is 80% S and 20% R, what is the specific rotation of the mixture?
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
This content is for registered users only.
By joining Chemistry Steps, you will gain instant access to the answers and solutions for all the Practice Problems including over 20 hours of problem-solving videos, Multiple-Choice Quizzes, Puzzles, and the powerful set of Organic Chemistry 1 and 2 Summary Study Guides.
Check also in Stereochemistry
- How to Determine the R and S configuration
- The R and S Configuration Practice Problems
- Chirality and Enantiomers
- Diastereomers-Introduction and Practice Problems
- Cis and Trans Stereoisomerism in Alkenes
- E and Z Alkene Configuration with Practice Problems
- Enantiomers Diastereomers the Same or Constitutional Isomers with Practice Problems
- Optical Activity
- Specific Rotation
- Racemic Mixtures
- Symmetry and Chirality. Meso Compounds
- Fischer Projections with Practice Problems
- R and S Configuration in the Fischer Projection
- R and S configuration on Newman projections
- R and S Configuration of Allenes
- Converting Bond-Line, Newman Projection, and Fischer Projections
- Resolution of Enantiomers: Separate Enantiomers by Converting to Diastereomers

I just registered to say thank you so much!!! I couldn’t be happier to find this website… Thank you!
Are there any other websites like this that you could recommend?
no
Very much educative
I appreciate your way in discussion of ee%