
In this post, we are going to learn a comparatively new rule, proposed by Henry Bent. Henry Bent (1926-2015) was an American physical chemist who was a well-known professor at North Carolina State University and at the University of Pittsburgh. The rule was stated recently in 1961 and it primarily talks about how the various atoms/ligands are oriented in space after hybridization.
Why is Bent’s rule needed?
In compounds with lone pairs or different substituents with trigonal bipyramidal(TBP) geometry, determining the correct structure can be difficult. It becomes mandatory to figure out the exact positions of the substituents or lone pairs, to determine the correct structure of the molecule.
e.g.– i)Consider PCl3F2 molecule.In this molecule, the central P atom is attached to five substituents ( three Cl and two F) and so it is sp3d hybridized (refer to the chart in an earlier post). This molecule has TBP geometry and can have the following structures –

ii) SF4 molecule has a lone pair of electrons. The structure of this molecule too can be written in two ways as follows-
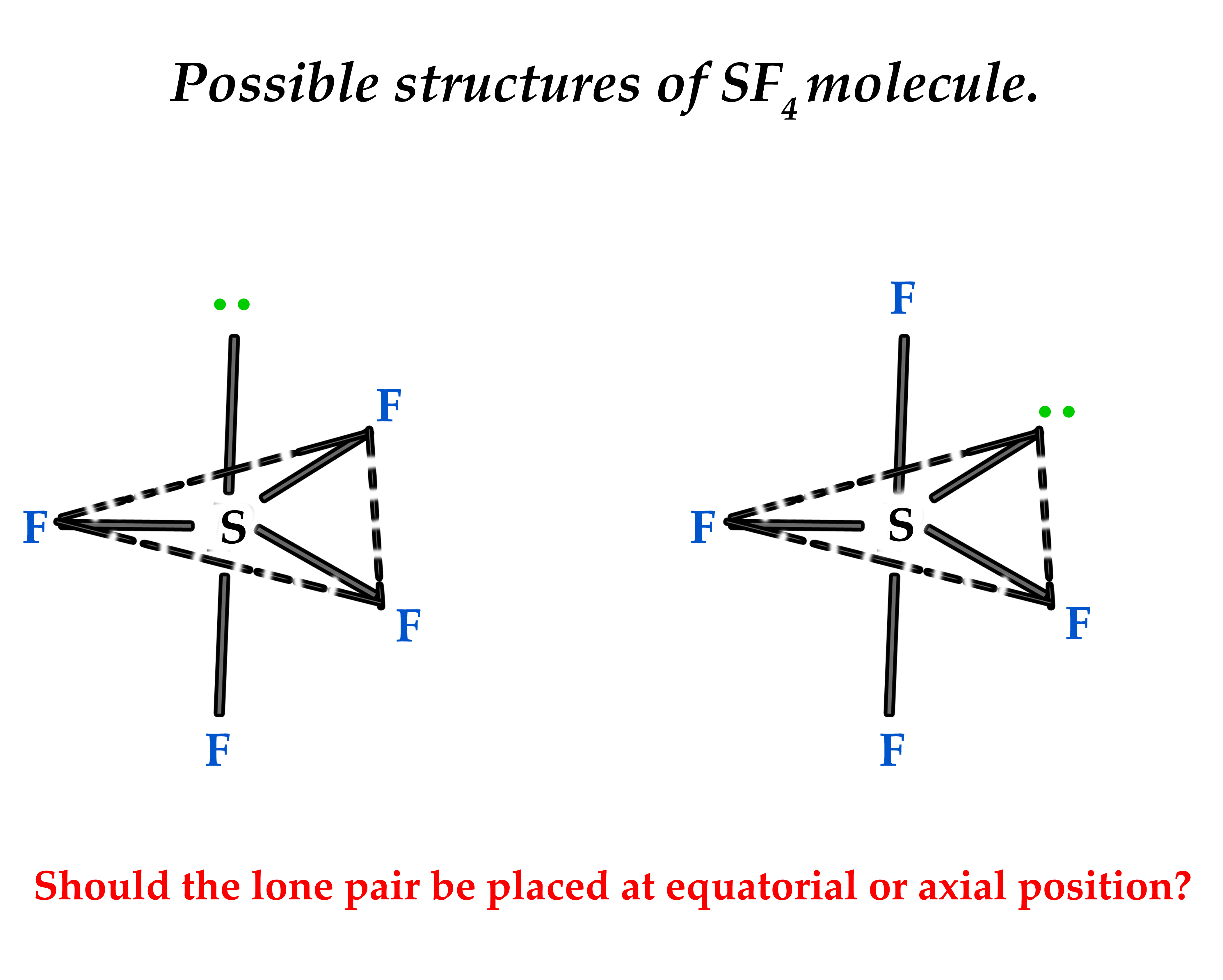
iii) Why does the C-F bond length vary in fluoromethane and difluoromethane?

Bent’s rule is the answer to all the above questions. The rule is helpful in explaining bond lengths, bond angles, the position of substituents in a molecule, and the structure of many molecules.
We have studied why substituents have their own preferences, in deciding which position they occupy, earlier in post 59. Now, we shall study that same concept again with a different approach.
Bent’s rule
” Atomic s character tends to concentrate in orbitals that are directed towards electropositive groups and atomic p character tends to concentrate in orbitals that are directed towards electronegative groups.”
We know that the s- orbital is closer to the nucleus and so as the s-character in a hybrid orbital increases, its electronegativity increases too.

So, it should come as no surprise that an orbital containing more %s character ( more electronegative) will prefer to be directed towards more electropositive groups. This is because – an electronegative group and an electronegative substituent will repel each other more, thus increasing the overall energy of the system. So, an electronegative hybrid orbital ( with more %s character) will always prefer to be directed at an electropositive group. e.g. sp hybrid orbital


A hybrid orbital with lesser or no s- character can easily be directed towards an electronegative substituent. e.g. p- orbital.
In our next post, we shall apply this rule to various molecules and study its consequence. Till then,
Be a perpetual student of life and keep learning..
Good Day!
Image Source –