Abstract
Seizures and epilepsy can result from various aetiologies, yet the underlying cause of several epileptic syndromes remains unclear. In that regard, autoimmune-mediated pathophysiological mechanisms have been gaining attention in the past years and were included as one of the six aetiologies of seizures in the most recent classification of the International League Against Epilepsy. The increasing number of anti-neuronal antibodies identified in patients with encephalitic disorders has contributed to the establishment of an immune-mediated pathophysiology in many cases of unclear aetiology of epileptic syndromes. Yet only a small number of patients with autoimmune encephalitis develop epilepsy in the proper sense where the brain transforms into a state where it will acquire the enduring propensity to produce seizures if it is not hindered by interventions. Hence, the term autoimmune epilepsy is often wrongfully used in the context of autoimmune encephalitis since most of the seizures are acute encephalitis-associated and will abate as soon as the encephalitis is in remission. Given the overlapping clinical presentation of immune-mediated seizures originating from different aetiologies, a clear distinction among the aetiological entities is crucial when it comes to discussing pathophysiological mechanisms, therapeutic options, and long-term prognosis of patients. Moreover, a rapid and accurate identification of patients with immune-mediated epilepsy syndromes is required to ensure an early targeted treatment and, thereby, improve clinical outcome. In this article, we review our current understanding of pathogenesis and critically discuss current and potential novel treatment options for seizures and epilepsy syndromes of underlying or suspected immune-mediated origin. We further outline the challenges in proper terminology.
Similar content being viewed by others
Identifying immune-mediated seizures is a key challenge in clinical practice. |
Antiseizure medications (ASMs) often fail to stop immune-mediated seizures and are usually used for symptomatic control. However, once the underlying immune-mediated mechanism is correctly identified and treated, seizures often subside. |
Novel immunomodulatory treatments are emerging as we gain more insight into immunopathophysiology. These therapies show promising effects and rapid initiation of immunomodualtion in immune-mediated seizures is crucial for outcome. |
1 Introduction
Epilepsy affects approximately 65–70 million people worldwide [1, 2]. It is estimated that the aetiology of one-third of all epilepsies in adults remains unknown [3] posing a significant diagnostic and therapeutic challenge as the treatment remains restricted to antiseizure medication (ASM). Overall, it is estimated that about 5% of focal epilepsy of unknown cause without clinically suspected autoimmune encephalitis may be immune-mediated [4,5,6,7,8].
In that regard, immunomodulatory treatment is increasingly seen as a treatment option in some drug-resistant epilepsy syndromes supporting a potential immune aetiology of epilepsy in these patients. Indeed, mounting evidence suggests that the immune system—in particular autoantibodies directed against neuronal cell surface proteins, intracellular antigens, receptors, or ion channels [9,10,11]—plays a significant role in the pathogenesis of a subset of these treatment-refractory epilepsy syndromes. In the last years, the significance of such antibodies and their pathogenicity has been increasingly elucidated, and the understanding of the underlying immune-mediated mechanisms is steadily growing. However, there are still questions surrounding the significance of such antibodies, their pathogenicity, and the underlying immune-mediated mechanisms. Moreover, immune-mediated seizures in the context of autoimmune encephalitis are highly prevalent, but they do not fulfil the diagnostic criteria of epilepsy.
In this article, we will outline the challenges of terminology, shed light on common and distinct pathophysiological mechanisms, and critically discuss current and novel therapeutic options in seizures and epilepsy syndromes of underlying or suspected immune-mediated origin.
2 Terminology
Given the overlapping clinical presentation of immune-mediated seizures originating from different aetiologies (Fig. 1), i.e., on the one hand, acute seizures in the context of immune-mediated diseases and, on the other hand, chronic predisposition to seizures due to an underlying immune-mediated disorder, it is important to clarify the current terminology, as it allows for a specific, targeted treatment. It has been proposed to differentiate between (1) acute symptomatic seizures secondary to autoimmune encephalitis (ASSAE), where diagnostic criteria are well defined and early immunotherapy is standard and (2) autoimmune-associated epilepsy (AAE) [12, 13], a disorder which so far poses many open questions.
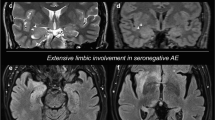
Overview of different aetiologies and overlapping pathophysiological mechanisms of autoimmune encephalitis (AIE), acute symptomatic seizures secondary to autoimmune encephalitis (ASSAE), autoimmune-associated epilepsy (AAE) and epilepsy leading to seizures. Aetiologies of immune-mediated seizures can be grouped into two main categories, (1) paraneoplastic (e.g., in teratoma or small cell lung cancer), and (2) non-paraneoplastic encompassing (a) infectious (e.g. in herpes simplex 1 encephalitis or varicella zoster virus encephalitis), (b) systemic/CNS autoimmune disorders (e.g., in multiple sclerosis or systemic lupus erythematosus), and (c) genetic predisposition (e.g., HLA-associated anti-LGI1 encephalitis). All of the aetiologies can lead to common pathophysiological mechanisms, such as the production of pathogenic autoantibodies, inflammation and subsequent structural damage, which then manifest in seizures in the context of AIE, ASSAE, AE and epilepsy. Figure was created using Biorender.
Autoimmune encephalitis (AIE) is defined as a non-infectious immune-mediated inflammatory disorder of the brain parenchyma often involving the cortical or deep grey matter with or without involvement of the white matter and meninges [14]. The aetiology is generally divided into two categories: paraneoplastic and non-paraneoplastic.
Acute symptomatic seizures associated with ASSAE describe repetitive seizures occurring in the acute phase of AIE and are among the most relevant clinical manifestations in AIE [15]. A seizure in general is defined by the International League Against Epilepsy (ILAE) as a transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain [16]. Most frequently, seizures can be found in AIE with autoantibodies directed against the GABA receptor, however, they also occur in other types of AIE. In anti-leucine-rich glioma-inactivated 1- (LGI1-), -GABAAR-, -GABABR- and -N-methyl-D-aspartate receptor- (NMDAR-) AIE prevalence of seizures range from about 76% to 100% [17]; the occurrence was lower in anti-AMPAR- and anti-GFAP-AIE with 31% and 28%, respectively [18] (Table 1). Usually, patients with AIE and seizures respond well to immunotherapy and ASM can be discontinued after immunomodulatory treatment [19]. The fact that persistent seizure-freedom can be obtained after immunotherapy (with or, in some cases even without ASM), contradicts the conceptual definition of epilepsy as an enduring predisposition to generate epileptic seizures and by the neurobiological, cognitive, psychological, and social consequences of this condition as defined by the ILAE [16] and argues that the term epilepsy is often wrongfully used in seizures associated with AIE.
In contrast, AAE describes unprovoked seizures following an autoimmune-mediated central nervous system (CNS) disease making the patient prone to seizures as a result from ongoing autoimmune processes, structural brain changes or a combination of both. Autoimmune-associated epilepsy is often falsely used to describe patients with AIE presenting with seizures. Autoimmune-associated epilepsy patients generally do not present the clinical features of acute AIE, but can test positive for neural autoantibodies, and do not respond well to immunotherapy. In this context, the chronic predisposition to seizure generation, despite immunomodulatory therapy, fits the current definition of epilepsy [13].
Even though it is crucial to acknowledge this distinction as it not only holds differing pathophysiological mechanisms, but also therapeutic consequences, the distinction will not be maintained throughout this review as this is a new concept and shared as well as divergent pathophysiological mechanisms are still to be understood.
3 Aetiology and Pathophysiology of Autoimmune-mediated Seizures and Epilepsy
The identification of the underlying aetiology of the seizure syndrome is a major aspect for any classification scheme to provide a directive for clinical practice. In general, the ILAE proposes to group epilepsy in six different aetiologies: structural, genetic, infectious, metabolic, immune, and unknown—the immune aetiology being recognized only since 2017 by the ILAE as a distinct category [20], however, the aforementioned distinction between ASSAE and AAE has not yet been included in the ILAE guidelines. In the following, we will discuss the aetiology and pathomechanisms of seizures in different immune-mediated contexts (Fig. 1).
3.1 Aetiology and Pathophysiology of Seizures in Autoimmune Encephalitides
3.1.1 Pathophysiology
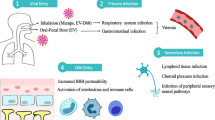
It is well accepted that both B and T cells contribute to the autoimmune processes in AIE and subsequent ASSAE or AAE (Fig. 2). Antigen-presenting cells present membranous or intracellular antigens, which are released following neuronal destruction or apoptosis by tumours, pathogens, or other mechanisms, which in turn prime T cells to secrete B cell-activating cytokines, such as interleukin-6 (IL-6). IL-6 has a variety of functions including the differentiating of B cells into antibody-producing plasma cells, which in turn means that a dysregulation of IL-6 can result in an overproduction of (pathogenic) antibodies leading to subsequent antibody-dependent cell-mediated cytolysis (ADCC) by activated macrophages, complement activation, and perforin (C5a-C9)-mediated cytolysis [21, 22]. CD8+ cytotoxic T cells attack (mostly intracellular) peptides that antigen-presenting cells have linked to MHC-I complex molecules. Both antibody- and T cell-mediated attacks finally cause neuronal dysfunction and/or degeneration [23], inflammation, and structural damage resulting in elevated susceptibility to seizures.
Overview of pathophysiological mechanisms in autoimmune encephalitis (AIE) and related therapeutic targets on the horizon. Antigens are released following either peripheral cell destruction by triggers such as apoptosis by pathogens (mostly viral), by tumours, systemic autoimmune disease or genetic predisposition (1) or direct neuronal destruction by pathogen invasion through impaired blood brain barrier (BBB) into the cerebrospinal fluid (CSF) (6). Antigen presenting cells (APCs) recognise the antigens (2), prime T cells to secrete B cell-activating cytokines (3). In turn, B cells differentiate from CD19+/CD20+ B cells into CD138+ plasma cells (4), which produce and secrete autoantibodies (5). Both antibody- and T cell-mediated processes lead to neuronal dysfunction and degeneration (7). Novel therapeutics agents (pink boxes) on the horizon targeting the above described patho-mechanistic processes, including B cell-depleting agents [anti-CD19 (inebilizumab), anti-CD20 (rituximab, ocrelizumab, ofatumumab), anti-CD38 depleting therapy (daratumumab)], agents targeting pathogenic antibodies/plasma cells and downstream mechanisms [anti-FcRn antibody resulting in IgG degradation (rozanolixizumab, efgartigimod-α), anti-C5 complement antibody (eculizumab), proteasome inhibitor (bortezomib)], agents that are targeting or modifying cytokines [anti-IL-6R antibody (tocilizumab, satralizumab), anti-IL-1R antibody (anakinra), anti-IL-1ßR antibody (canakinumab), low-dose IL-2 therapy and Janus kinase inhibitor (tofacitinib)] and others [CD80/CD86 ligand blocker (abatacept), anti-TNF-α receptor antibody (e.g., adalimumab)]. Figure was created using Biorender.
Autoimmune encephalitis is mediated by the production of pathogenic autoantibodies and autoreactive T cells targeting neuronal surface or intracellular structures (Fig. 2). In about 40% of encephalitides the cause remains unknown [24]. Autoimmune encephalitis can either be associated with tumours—the so called “paraneoplastic” AIE—or “non-paraneoplastic”. Antibodies directed against intracellular antigens are associated with paraneoplastic AIE and are therefore called “onconeural antibodies”. These antibodies are thought not to be directly pathogenic; however, their action is mainly mediated through cytotoxic T cells resulting in neuronal loss and an overall worse outcome and response to immunotherapy [25].
Antibodies directed against cell surface antigens are usually associated with non-paraneoplastic aetiology in AIE. Non-paraneoplastic AIE are mostly antibody-mediated. Antibody-mediated internalization of neuronal surface receptors or blocking of receptor function [26, 27] results in reversible neurologic dysfunction, and, hence, an overall better treatment response to immunotherapy.
Autoantibodies of the immunoglobulin G (IgG) isoform have been most extensively studied in AIE. In anti-NMDAR AIE the predominant IgG subclasses are IgG1 and IgG3. IgG1 and IgG3 exhibit high complement activation and high affinity for Fc receptors, which results in cell-mediated cytotoxicity, complement activation, induction of tissue damage and internalization of the antigen. This stands in contrast to the IgG4 subclass, which exhibits low affinity for complement and Fc receptors largely resulting in mild, non-complement–meditated inflammation and requiring other immunomodulatory treatment. Predominance of autoantibodies of the IgG4 subclass has been described in, e.g., anti-LGI1 AIE and anti-CASPR2 mediated limbic encephalitis [28,29,30,31].
These data underscore differing pathophysiological mechanisms regarding predominant Ig subclasses, an understanding which is crucial for guiding treatment decisions. As an example, eculizumab, an anti-C5–complement protein antibody [32], could serve as a possible immunotherapeutic target in AIE with predominant complement-activating Ig subclasses, such as IgG1 and IgG3.
3.1.2 Immune Mechanisms Underlying Seizures
Seizures are frequent in AIE. However, the overall risk of developing autoimmune-associated epilepsy is generally small with a higher risk in cases with autoantibodies directed against intracellular antigens compared to neuronal cell surfaces [10].
In anti-NMDAR-AIE, the autoantibodies block the NMDAR, which leads to the internalisation of the receptor and a lack of glutamatergic neurotransmission, which may result in a decrease of excitatory input on inhibitory interneurons in the limbic structures and basal ganglia, eventually causing seizures and excessive hyperkinetic movements, e.g., orofacial dyskinesia [33].
The pathognomonic, a few seconds-lasting, seemingly tic-like, faciobrachial dystonic seizures (FBDS) in anti-LGI1-AIE are semiologically quite different from common seizure types of frontal and temporal origin, and involvement of subcortical structures, especially basal ganglia has been observed in imaging studies [34, 35]. The LGI1 protein is largely expressed in the cortex and hippocampus adjacent to the voltage-gated potassium channel Kv1.1 [36]. The protein links its presynaptic receptor ADAM23 with its postsynaptic receptor ADAM22. Anti-LGI1-antibodies recognising the leucine-rich domain lead to internalisation of the whole protein complex while those antibodies recognising the epitempin domain hinder the docking of the protein with both receptors, ADAM22 and 23. The latter may lead to hyperexcitability and as a clinical result to seizures, and the former induces memory impairment [37, 38]. In addition, recent work in rats showed that blocking Kv1.1 potassium channels by these antibodies launches a cascade of alterations in neuronal excitability and synaptic activity resulting in large suprathreshold membrane depolarisations forming the paroxysmal clinical and EEG activity [39].
In anti-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-AIE, the autoantibodies cause a decrease of cell surface receptor clusters by internalisation and degradation. Single cell recordings revealed that the antibodies reduce AMPA receptor-mediated currents, especially inhibitory synaptic currents, while the intrinsic excitability of neurons and short-interval firing increased. These mechanisms may clinically lead to lower seizure threshold and repetitive AASAE [40, 41].
Another ictogenic pathomechanism associated with antineuronal antibodies is allosteric blocking of receptors or their subunits, like in anti-GABA-A receptor-associated AIE. Hereby, the targeting of the α3-subunit of GABAA receptors conceivably decreases the inhibitory effect of these receptors on neuronal excitability by reducing the inhibitory postsynaptic potentials (IPSPs) while leaving the excitatory postsynaptic potentials (EPSPs) unaffected. This profound imbalance leads to severe cortical hyperexcitability and ultimately to clinical seizures and often status epilepticus [42, 43]. Isolated blocking of the α1 subunit inhibits the ligand binding by direct antagonism, while antibodies targeting the α1γ2 subunits block the receptor function by allosteric antagonism [44, 45]. Whilst anti-GABAA receptor antibodies act on postsynaptic currents, the antibodies directed against the GABAB receptors influence presynaptic mechanisms and—unlike in anti-NMDAR-, -AMPAR- and -GABAAR-AIE—do not lead to internalisation of the receptors, but reduce excitability of neurons in the mesial temporal lobe, thus, leading to fewer seizures, but more profound memory deficits [46].
A distinct pathophysiological mechanism underlies anti-glutamic acid decarboxylase (GAD) AIE. Glutamic acid decarboxylase catalyses the direct transformation of the strongest excitatory neurotransmitter glutamic acid into the strongest inhibitory neurotransmitter gamma-amino-butyric acid (GABA) with pyridoxine (vitamin B6) as a cofactor. The enzyme is located intracellularly, but, unlike the onco-neuronal antibodies binding in the perinuclear space, is situated in the cytoplasm next to the endoplasmic reticulum. The GAD65 antibodies may cause seizures not only by the accompanying immune mechanisms, but essentially by suppressing GABAergic inhibitory transmission [47]. This will induce failure of converting excitatory glutamate into inhibitory GABA and therefore create a permanently hyper-excitatory state with reduced availability of GABA in the synaptic cleft [48] and elevated amounts of presynaptic glutamate, eventually resulting in limbic AIE, acute seizures as well as chronic pharmaco-resistant (autoimmune-associated) epilepsy [49, 50]. The exact mechanisms and their extent to these disorders remain to be elucidated. It is difficult to explain how the antibodies gain access to the intraplasmatically located enzyme GAD and influence its function. Other authors are puzzled by the variety of anti-GAD-associated disorders (epilepsy, limbic encephalitis, cerebellar degeneration, stiff-person syndrome, psychosis, etc); however, these disorders show different titres of these antibodies and, in particular, the antibodies of a specific disorder recognise different epitopes on the large 65kD protein [51, 52].
3.1.3 Aetiology
While the aetiology of the majority of non-paraneoplastic AIE remains unclear, in rare cases they can be the result of complex immune-mediated processes following viral infection, where viral-induced neuronal damage leads to a secondary autoantibody production with subsequent AIE most prominently described for anti-NMDAR and anti-GABAB-receptor AIE following HSV1 infection [53,54,55,56,57]. Similar processes have been described for varicella zoster virus (VZV) [58, 59] and Epstein Barr Virus (EBV) infection [60] (Fig. 2). More recently, cases of seronegative AIE or AIE with positive anti-Caspr2 antibodies following SARS-CoV2 infection have been reported [61]. Although seizures were associated with SARS-CoV2 infections [61, 62], most case reports attribute the seizures to the acute viral infection in the context of the release of pro-inflammatory cytokines lowering seizure threshold during the infection, mechanisms that are not SARS-CoV2 specific [63].
A few studies have looked at genetic predisposition in AIE: HLA subtype associations have been shown in anti-LGI1/anti-NMDAR [64, 65], anti-GAD65 [66] and anti-CASPR2 encephalitis [67]. One study by Kim et al. investigated patients with anti-NMDAR AIE as a control group, where an HLA association was not observable [68]. Future studies powered to detect genetic signatures will give a clearer understanding on the underlying genetic predisposition of AIE.
Regarding the compartmentalisation of the immune response, autoantibodies can be found either in serum, cerebrospinal fluid (CSF), both or neither (Fig. 2). It remains a matter of debate whether intrathecal synthesis of pathogenic autoantibodies in AIE leads to subsequent seizures or whether seizure-mediated blood-brain barrier (BBB) breakdown could result in antigen exposure and production of CNS-directed autoantibodies [4, 69]. On the other hand, intrathecal production of antibodies by plasma cells in AIE potentially points to a compartmentalised CNS process independent of BBB disruption, which may explain why some patients present with severe clinical deficits but have undetectable serum autoantibodies and the limited effectiveness of therapy with intravenous immunoglobulin (IVIG) and plasma exchange (PLEX) (showing higher effectiveness in other systemic antibody-mediated autoimmune diseases such as myasthenia gravis), which does not act directly on the CNS compartment [70].
3.2 Aetiology and Pathophysiology of Seizures in Systemic or CNS-related Autoimmune Diseases
Seizures are often associated with systemic autoimmune diseases (SAD) with or without direct involvement of the CNS, such as systemic lupus erythematosus (SLE), diabetes mellitus type 1, myasthenia gravis, sarcoidosis, Crohn's disease, celiac disease, or Hashimoto’s encephalopathy, to name only a few. Patients with SAD in general have shown to have about a 2.5-fold increased risk of epilepsy. Specifically, patients with SLE have up to a 4.5-fold risk of epilepsy. In turn, patients with epilepsy have been shown to have a 2.5- to 3.8-fold increased odds ratio of SAD in a meta-analysis by Lin et al. and a study by Ong et al. [71, 72].
Different mechanisms of epileptogenesis seem likely: hereby, the adaptative immune system plays a minor role since the autoantibodies involved in these diseases may not directly target neuronal structures. Thus, the main effector cells in epileptogenesis associated with SAD are those of the innate immune system. While its effector cells (microglia, NK cells) and cytotoxic T cells also may play a minor role, the general alterations in and exposure to increased amounts of cyto- and chemokines seem to be the major causes of icto- and epileptogenesis [73,74,75]. A systemic immune activation generating a pro-inflammatory state with altered cytokine and chemokine secretion in SAD is likely to result in a lowered seizure threshold [76] by directly altering expression and function of receptors of inhibitory and excitatory neurotransmitters or by launching cascades of molecular signalling, which lead to translational and post-translational changes of expression of neurotransmitters and/or their receptors. The main components of these mechanisms are the induction of interleukin-1 receptor-1- (IL-1R1-) signalling by interleukin-1β- (IL-1β-) and toll-like receptor-4 (TLR4)- signalling by high mobility group box1 (HMGB1). The subsequent downstream activation of kinases leads to activation of transcription of inflammatory genes and/or post-translational changes of neuronal proteins, like channels and receptors [75]. While short-term exposure of neurons to IL-1β leads to a reduction of voltage-gated sodium currents, longer exposure (hours or days) results in a marked increase and can cause hyperexcitability [77]. The same, time-dependent effects of IL-1β are observed on GABA-A receptor mediated currents in ganglioglioma [78]. It also has been shown that IL-1β enhances expression of NMDA receptor subtype 2B leading to an increased Ca2+ influx and hyperexcitability. Similarly, activation of tumour necrosis factor receptor 1 (TNFR1 or p55) by tumour necrosis factor α (TNFα) influences- like IL-1β- sodium, potassium, and calcium currents, and upregulates the expression of NMDA type 1 and AMPA GluR1 and GluR2 receptors, thereby increasing Ca2+ influx, which in sum leads to a state of hyperexcitability [76].
In addition, the unspecific inflammatory reactions mediated by cyclooxygenase-2 (COX-2) transformation of arachidonic acid into prostaglandins E and F cause neuronal hyperexcitability by substantial Ca2+release from activated astrocytes [73, 79]. Newer data reported another important target in the prostanoid pathway: the enzyme monoacylglycerol lipase (MAGL) hydrolyses the endocannabinoid 2-arachnoidonoylglycerol to arachidonic acid; inhibition of MAGL reduced neuroinflammatory markers in a mice model and stopped diazepam-resistant status epilepticus (SE) [80]. Neuroinflammation is also reciprocally associated with oxidative stress. Hereby, the activation of toll-like receptors produces reactive oxygen and nitrogen species. Conversely, these compounds induce the NLRP3 inflammasome by activating the antioxidant protein thioredoxin [81, 82].
Furthermore, seizures in SAD could stem from direct neuronal damage through the formation of immune complexes (sarcoidosis or Hashimoto's encephalopathy), from vascular changes, especially vasculitis of cerebral vessels and subsequent ischaemic strokes, as well as perivascular haemorrhage, from dysregulation of electrolytes or from shared genetic predisposition [83].
Blood-brain barrier disruption is another, rather unspecific, effect of immune-mediated neuroinflammation. Pro-inflammatory chemo- and cytokines gain direct access to neurons, glial cells, (damaged) endothelial cells and pericytes [84,85,86,87], and the cyto-pedesis of immune cells, especially T and NK cells evoke and maintain neuroinflammation, cell necrosis, and gliosis of cerebral tissue. In addition, the extravasation of the important serum protein albumin results in hyperexcitability by activating transforming growth factor-β receptor 2 and its downstream signalling, which leads to substantially altered astrocytic function, i.e., downregulation of the glutamate transporters GLT1 and GLAST, the aquaporin 4 channel, and the inwardly rectifying potassium channel Kir4.1, eventually summing up in increased extracellular K+ and glutamate, as well as disturbed water homeostasis [88,89,90]. It is important to mention that all these mechanisms may remain activated throughout the persistence of SAD-associated seizures in a self-reproducing manner and, consequently, maintain a vicious circle.
3.3 Aetiology and Pathophysiology of Epileptic Syndromes with Suspected Immune Aetiology without Known Associated Antibodies—the Example of Rasmussen Encephalitis
There are several very rare epilepsy syndromes, including West syndrome, Lennox-Gastaut syndrome, Landau Kleffner syndrome, and Rasmussen’s encephalitis (RE), which are often of unknown origin and in some cases associated with structural or genetic changes. Although an autoimmune mechanism has been suspected in some cases, no known associated autoantibodies have yet been detected. However, an autoimmune origin is widely accepted in RE. Patients affected by RE, usually children aged between 3 and 10 years (but onset at both younger and even adult age are reported), start to suffer from hemispheric cortical inflammation (most pronounced in the motor cortex), which results in absolutely treatment-refractory focal epilepsy with mainly motor epilepsia partialis continua and progressive neurological and, later on, cognitive decline [91]. Initially, the disease seemed to be caused by antibodies directed against the glutamate receptor subtype-3 (anti-Glu3) [92], but this association could not be reliably reproduced in a follow-up study [93]. The same is true for other anti-neuronal autoantibodies (anti-nicotinic alpha-7-, anti-Munc-18- and the anti-GluR2/3 (AMPA-R-)-Abs), found in some patients; they currently are considered merely an epiphenomenon of the massive inflammatory processes exposing neuronal antigens, which may serve as epitopes for antigen-presenting cells [94]. The disorder is mainly mediated by cytotoxic granzyme B+ CD8 T cells, NK cells, and activated microglia expressing IL-1α, IL-18, and caspase-1 [95, 96], which lead to severe neuroinflammation, necrosis [97] and caspase-1-mediated pyroptosis [98]. Recent findings examining 27 surgical specimens with control autopsies and people with non-RE-related epilepsy by whole exome sequencing did not reveal common pathogenic variants in RE but detected many variants of unknown significance in genes associated with severe epilepsies, as well as an accumulation of rare allelic HLA variants (HAL-DRB1 and HLA-DQA2) in > 25% of RE patients, which are represented in < 5% of the general population. Ribonucleic acid (RNA) sequencing additionally detected significant activation of the crosstalk between dendritic and NK cells, of neuroinflammatory signalling, and of phagosome formation [99]. This exciting new data may pave the way for new therapeutic alternatives in RE, especially TNFα blockers, and caspase-1 inhibitors, like belnacasan (VX-765).
4 Clinical Presentation and Diagnosis
4.1 Clinical Presentation and Diagnosis
Since clinical presentation and diagnosis is not the key focus of this review, we would like to refer to other detailed reviews in this regard [14, 15, 100, 101]. Here, we will solely provide a brief overview mainly focusing on the frequency of seizures and EEG hallmarks of different autoantibody types.
Autoimmune encephalitis presents clinically heterogeneously, often with subacute memory decline, changes in consciousness, seizures in combination with autonomic dysregulation, psychiatric symptoms, and other neurological deficits. Although varying in presentation, there are a few pathognomonic signs and symptoms, such as faciobrachial dystonic seizures in anti-LGI1-AIE [102], extrapyramidal orofacial dyskinesia in anti-NMDAR-AIE [103] and refractory status epilepticus in anti-GABAA-AIE [104] (Table 1).
Generally, AAE can be suspected if there are pharmaco-resistant seizures combined with or without suspicion of an underlying autoimmune disease or if there are seizures hard to control with ASM that cannot be explained other than caused by an immune-mediated process. Diagnosis is based on autoantibody testing in serum as well as in cerebrospinal fluid (CSF), magnetic resonance imaging (MRI) and electroencephalogram (EEG), as well as screening for underlying oncological cause [tumours are less common in AIE with neuronal surface antibodies, but frequent in AIE with intracellular antibodies (onconeural antibodies)] [105]. Electroencephalogram is informative to detect subclinical seizures, convulsive/non-convulsive status epilepticus or serve as a diagnostic tool if brain MRI does not show pathological changes. In a study by Steriade et al., it was shown that up to 58% of patients with AIE experience subclinical seizures [106]. Interestingly, apart from faciobrachial dystonic seizures, which typically occur in anti-LGI1-AIE and “the extreme delta brush” in anti-NMDAR-AIE, EEG changes are similar across different autoantibodies (Table 2).
In addition, [18F]-FDG-PET imaging, especially the decreased cortex/striatum ratio, seems to provide supporting evidence for the presence of AIE in patients with clinical suspicion of AIE but normal brain MRI [107] and is also recommended to be performed in this situation [14].
Diagnostic scoring systems include the Antibody Prevalence in Epilepsy and Encephalopathy (APE) and APE2 score [8, 15, 108], the APES [109] and ACES score [8]; however, further elaboration lies beyond the scope of this review.
4.2 Acute Symptomatic Seizures and Possible Progression to Epilepsy
Rada et al. investigated patients with seizures and neuronal autoantibodies over a time frame of ≥ 3 years and found that seizures with positive surface autoantibodies are mostly ASSAE with a good chance of seizure freedom after combining ASM and additional immunotherapy. Also, titres of surface antibodies- but not intracellular antibodies- decreased over time and correlated with clinical course [110].
In another study by Ilyas-Feldmann, 1 year after AIE diagnosis seizure freedom occurred more frequently in patients with neuronal surface antibodies in comparison with intracellular antibodies [19]. Chen et al. defined post-AIE epilepsy as one unprovoked seizure ≥ 6 months after hospital discharge finding nearly 40% of AIE patients included in his study which met these criteria. Of these, the majority had autoantibodies directed against intracellular targets [111].
These findings emphasise that neuronal surface autoantibodies are ictogenic, but not epileptogenic, rendering the CNS in a state prone to seizures, but once the underlying condition is treated by a targeted therapy the seizures subside. However, in patients with presence of intracellular antibodies unprovoked seizures often occur, despite treatment with ASM and immunotherapy, and although seizure frequency decreases over time, patients rarely attain seizure freedom and often develop epilepsy [110].
5 Pharmacological Treatment
As we have previously discussed, AIE often presents with seizures (ASSAE), often refractory to ASM, and treatment is mainly directed to the underlying cause –the dysregulation of the immune system. Once the dominant type of immune mechanism is identified and properly treated, most seizures subside [8] (except those associated with anti-GAD65 antibodies [49]). Therefore, immunomodulation is of major importance in controlling seizures of immune-mediated mechanisms. The following section will focus on proposed practice recommendations of acute and long-term treatment and will review modes of action of potential novel immunotherapeutic agents (Fig. 2, Table 3).
We would like to point out that the following paragraphs (especially 5.1–5.4) are merely recommendations based on already available literature, as there are no evidence-based guidelines available. The second part on novel immunomodulatory approaches is an overview of potential treatment options on the horizon based on underlying pathophysiological mechanisms; however, they are neither approved, nor based on a guideline and are mainly derived from single case reports/ series or from authors’ clinical experience.
As in many other severe acute diseases with the propensity to relapse and/or chronification, the terms “first-line” and “second-line treatment” have been increasingly used without a clear definition, but they are mentioned in the first large series of AIE patients [112] supported by expert opinion [113] and then widely used and investigated [114]. While corticosteroids, IVIG, and PLEX immunoadsorption were considered to be first-line treatments, second-line treatments include many immunomodulating agents administered for achieving three goals: (1) to intensify the treatment in cases with insufficiently controlled AIE, (2) to enable a continuous corticosteroid-sparing maintenance therapy, and (3) to prevent relapses of the disease.
5.1 Treatment of AIE
5.1.1 Acute Treatment in Suspected AIE
Early initiation of treatment correlates with better outcome for patients. Therefore, empiric treatment in suspected AIE is usually started before laboratory results for antibody testing return [15, 105]. The initial treatment of choice is high-dose intravenous corticosteroids. If corticosteroids are contraindicated or treatment response is not sufficient, therapy with IVIG or PLEX can be considered [14]. Starting a dual therapy [e.g. (1) corticosteroids + IVIG or (2) corticosteroids + PLEX] from the onset of disease can be considered in patients with severe initial presentations. In paraneoplastic AIE with autoantibodies directed against intracellular antigens, pathogenesis is cell- and not antibody-mediated, making IVIG an ineffective treatment [14, 115]. Plasma exchange may be especially effective in cases where high antibody titres are present as it clears blood of pathogenic antibodies [116]. An even more refined treatment strategy consists of immunoadsorption where a specifically prepared adsorption column targeting the specific pathogenic antibody of the patient binds and removes these antibodies from the serum. This method showed better efficacy than PLEX with fewer adverse effects in some small studies [117, 118].
5.1.2 Escalation Treatment in Suspected AIE
Second-line agents such as rituximab (RTX) or cyclophosphamide (CTX) can be implemented if no clinical improvement is observed 2–3 weeks after the initiation of treatment. Rituximab is thought to be more effective in patients with antibody-mediated AIE [14], in contrast, CTX can be the first choice in cell-mediated AIE where autoantibodies are usually directed against intracellular antigens [119]. If this does not lead to clinical improvement, experimental approaches with IL-6 inhibitors (tocilizumab), low-dose IL-2 therapy or proteasome inhibitors (bortezomib) can be evaluated (later covered in more detail under the section of potential novel immunomodulatory agents).
5.1.3 Symptomatic Treatment of Seizures During AIE
Treating the underlying condition by immunotherapy in immune-mediated epileptic syndromes is crucial for seizure control in AIE. Antiseizure medication can be considered as an add-on therapy. Although there are no clear recommendations for the choice of ASM in AIE, at least in anti-LGI1 AIE, the inhibition of voltage-gated sodium channels, for example with carbamazepine, was demonstrated to be more effective than levetiracetam in reducing seizures [120]. In general, when treating AIE-related seizures, one should avoid ASMs that are influenced by the hepatic cytochrome 450 (CYP450) enzyme system. Phenytoin, for example, is mainly metabolised through the CYP450 enzyme system isoforms CYP 2C9 and 2C19 [121] and reduces plasma concentration of corticosteroids via enzyme induction while corticosteroids reduce the availability of phenytoin by the same mechanism [122, 123]. Carbamazepine and rifampicin count as the most powerful CYP450 inducers [124, 125], this also applies to phenobarbital, which shows a slightly weaker effect [126]; in the context of AIE and AAE, this is particularly important when using corticosteroids, doses of which need to be higher by 50–60% and be cautiously tapered. Valproate, on the other hand, is a CYP450 inhibitor and may enhance the effect of certain medications [127]. Moreover, a recent review by Berman et al. discusses drug-drug interactions of ASM and therapies using monoclonal antibodies: although most combinations do not need special precautions, in some specific combinations, drug monitoring or dose adjustment is needed. It is important to know that pro-inflammatory cytokines, like IL-6 and TNFα, may reduce the expression of CYP450 isoenzymes, and therefore increase the levels of ASM subject to CYP450 metabolism. Contrarily, the application of antagonists of these cytokines, like tocilizumab for IL-6, may reduce, i.e., “normalize” the ASM level [128]. Therefore, potential drug interactions need to be kept in mind in patients showing limited therapeutic response when receiving ASM and immunotherapy. As a general rule, antiseizure medications that alter CYP 3A4/5 and CYP 2C9/19 expression (induction or inhibition), like carbamazepine, phenytoin, and to a lesser extent, ox-/eslicarbazepine, phenobarbital, valproic acid, and the new ASM cenobamate, as well as pure substrates of these CYP450 isoforms, like perampanel, zonisamide, and midazolam (all 3A4/5), and lacosamide and brivaracetam (both 2C19), may be used with some caution together with cytokine antagonising antibodies targeting IL-6 and TNFα [129]. Antiseizure medication, not subject to CYP450 metabolism, like levetiracetam, gabapentin, pregabalin, and lamotrigine, may be preferred in this situation, although levetiracetam has been shown inferior to sodium channel blockers in anti-LGI1-AIE [120]. Furthermore, lamotrigine with its mandatorily slow titration schedule might be a problematic choice in the acute situation of AIE.
5.1.4 Long-term Treatment and Prevention of Relapse in AIE
After high-dose immunotherapy, a subsequent bridging therapy should be initiated as prompt discontinuation of immunotherapy shows higher risk of relapse. Usually this is performed by oral administration of corticosteroids with a gradual corticosteroid taper. Alternatively, regular intravenous administration of methylprednisolone or IVIG can be considered.
Relapses occur in about 20–32% of all patients [108, 130, 131]. In general, antibodies directed against surface antigens have higher relapse rates than antibodies directed towards intracellular antigens, especially if the underlying tumour is treated accordingly [132]. Once a relapse occurs, long-term immunosuppression is indicated. Azathioprine, mycophenolate mofetil, and RTX can be considered as long-term treatment options. Maintenance therapy in a relapsing course of disease has been suggested by some authors for up to 3 years [132, 133].
5.1.5 Treatment of Paraneoplastic Comorbidity
In paraneoplastic AIE, treatment of the underlying neoplasm is crucial. In some cases, patients do not need additional immunotherapy as symptoms subside once the neoplasm is appropriately treated, i.e., by surgery, chemotherapy or radiation. Immune checkpoint inhibitors (ICIs) are used more and more in oncologic treatment. Unfortunately, treatment with ICI can be a cause of encephalitis or can contribute to the exacerbation of AAE, which is why it is advised to evaluate with treating oncologists if ICI should be discontinued [134].
5.1.6 RITE and RITE2 Score
The Response to ImmunoTherapy in Epilepsy (RITE) score can be used to predict treatment response to immunomodulation in patients with AE. It includes variables from the APE score as well as the timepoint of initiation of immunotherapy and the detection of antibodies directed against cell-surface antigens. A RITE score ≥ 7 predicts a favourable outcome (reduction in seizure frequency of at least 50% after initiation of immunotherapy) and is correlated with an early initiation of treatment and the presence of autoantibodies directed against membrane-bound epitopes [135].
The RITE score was later modified to the RITE2 score. Dubey et al. investigated the RITE2 score retrospectively in patients with neural autoantibody positivity or favourable immunotherapy outcome in order to validate the score, finding that a RITE2 score ≥ 7 had a sensitivity of 96% for favourable response to immunotherapy. Interestingly, a RITE2 score < 7 was associated with refractoriness to immunotherapy even among patients with neural-specific autoantibodies indicating that the RITE2 score might identify patients with pathogenic autoantibodies [136].
5.1.7 NEOS Score
The anti-NMDAR Encephalitis One-Year Functional Status (NEOS) score assesses neurologic function 1 year after diagnosis of anti-NMDAR-AIE. The score is associated with poorer functional status 1 year after diagnosis. Interestingly, a significant proportion of the patients with poor functional status (≥ 3 points) at 1 year after diagnosis recovered to good functional status (≤ 2 points) after 2 years of diagnosis. So rather than a score to predict outcome, the score could be used in clinical practice as a tool to evaluate initial course of clinical improvement [137].
5.2 Treatment of Autoimmune-associated Epilepsy
Since AAE is not a well-defined disease entity and pathophysiological understanding is still lacking, there is limited data available on treatment options for patients with suspected AAE. In a trial by Toledano et al. [138], 110 patients who reported seizures were screened. Twenty-nine patients met the criteria for “suspected autoimmune epilepsy”, i.e.,, presence of ≥ 1 positive neural autoantibody, personal/family history, or current signs of autoimmunity on examination and frequent and pharmacologically uncontrollable seizures. Seizure frequency was obtained by medical record review, converted to weekly rates in order to calculate post-baseline seizure frequency. A 6- to 12-week trial with intravenous corticosteroids, IVIG or both was initiated, and responders were defined as ≥ 50% reduction in seizure frequency (calculation of seizure quantification during the periods of time following initiation as stated above). The study concluded that 62% of patients were responders, of whom 34% attained seizure freedom and 52% improved with the first agent. This trial was not only proposed to justify further trials with immunotherapy in patients with suspected AAE, but again highlighted the considerable amount of AAE in patients presenting with seizures and probable underdiagnosing of these patients. The retrospective study of von Rhein et al. conversely looked at the effect of high-dose methylprednisolone on outcome and its prediction in 28 patients with pharmaco-resistant anti-neuronal antibody-negative temporal lobe epilepsy with additional features suggestive of an associated limbic encephalitis [clinical (cognitive decline, behavioural problems), MRI, inflammatory CSF, PET]. They observed seizure freedom in almost half of patients (46%) after 18 months of follow-up, but also worsening in one-third of patients. Unfortunately, outcome was not predictable [139]. Since then, no newer data or randomized controlled trials have become available on this very important and probably not rare clinical situation.
5.3 Treatment of Epileptic Syndromes with Suspected Immune Aetiology without Known Autoantibodies—the Example of Rasmussen Encephalitis
Epileptic syndromes with suspected immune aetiology without known autoantibodies respond well to immunomodulation while ASMs remain of limited use. Here we will elaborate further on RE as an example for an epileptic syndrome without known antibody target. While ASMs are ineffective in RE [91], some patients improve from the administration of corticosteroids [140], PLEX [141], IVIG [142] or tacrolimus [143] and azathioprine [144]. Other compounds used in single cases include RTX, natalizumab, methotrexate (MTX), and alemtuzumab. In accordance with new findings on the role of TNFα in icto- and epileptogenesis of RE mentioned above, some patients showed partial benefit from the TNFα antagonist adalimumab [145]. The most effective treatment in this desperate situation consists of functional hemispherectomy in children as early as possible since the plasticity of the infant brain may lessen the severe sequelae of the disease as well as of the surgical treatment [146].
5.4 Novel Immunotherapeutic Players on the Horizon
Emerging novel therapeutic agents targeting different players in the immune cascade have arisen by progressively deciphering underlying pathomechanisms in AIE (Fig. 2). Some of the following drugs are approved for other indications but are off-label for the treatment of AIE and immune-mediated seizures.
5.4.1 B Cell Depleting Agents
5.4.1.1 Rituximab (Anti-CD20 Antibody)
As mentioned, RTX—a B cell depleting therapy (BCDT) targeting the CD20 antigen on the surface of B cells—is already used for AIE as a second-line therapy. However, long-lived plasma cells are not targeted by RTX [147, 148], which may result in continuous production of pathogenic autoantibodies and could explain why some AIE are RTX-resistant. Novel BCDT do not only target CD19+ and CD20+ for B cell differentiation but also CD38+ and CD138+ leading to the suppression of plasma cell differentiation. Interestingly, diseases mediated by the IgG4 subclass, e.g., anti-MUSK antibody myasthenia gravis or pemphigus vulgaris, respond very well to RTX [149, 150], albeit for unclear reasons. It has been proposed that IgG4 production in IgG4-mediated diseases are not driven by long-lived plasmablasts or if they are, then potentially by CD20 expressing IgG4 plasmablasts [28, 151]. Considering these aspects, RTX may be a potent first-line treatment in patients with AIE who are primarily mediated by IgG4, like anti-LGI1-, anti-Caspr2-, anti-DPPX- and anti-IgLON5-AIE.
In general, a combination of B cell- and plasma cell-targeting therapies may represent a therapeutic regimen of therapy-refractory AIE as it would lead to a complete suppression of B cells and antibody production. However, data on efficacy and safety of such combination strategies are limited. It needs to be kept in mind that—like other BCDT—effects of long-term B cell suppression can lead to hypogammaglobulinemia, increased risk of infection/more severe course of infection as well as lessened response to vaccinations [152, 153].
5.4.1.2 Ocrelizumab and Ofatumumab (Anti-CD20 Antibody)
Ocrelizumab is a humanised anti-CD20 monoclonal antibody, which is used to treat relapse-remitting and primary progressive multiple sclerosis. By binding to an overlapping epitope, the mechanism of action is similar to RTX [154]. However, since it is derived from mostly human antibodies, it is suggested that ocrelizumab might have a better efficacy and an improved safety profile when compared to RTX [155] To date, there has been only one prospective, randomised, placebo-controlled trial aimed at investigating the role of ocrelizumab in patients with AIE (NCT03835728). Unfortunately, the study could hardly recruit patients, one patient with anti-NMDAR-AIE (IgG1-mediated) showed improvement, the other patient with anti-LGI1-AIE (IgG4-mediated) remained clinically stable, one received placebo. No adverse events were reported [156]. Another CD20-targeting therapy that may be evaluated as a potential immunotherapy in AIE is ofatumumab, a fully human anti-CD20 monoclonal antibody that is used to treat relapsing-remitting multiple sclerosis. To date no clinical trials exist using ofatumumab to treat AIE.
5.4.1.3 Inebilizumab (Anti-CD19 Antibody)
Inebilizumab is a humanised anti-CD19 monoclonal antibody that is approved in the USA for the treatment of neuromyelitis optica spectrum disorders (NMOSD) [157]. As it depletes CD19+ B cells, including plasmablasts and plasma cells, it is suggested to result in a sustained suppression of B cell expression. There is currently one trial ongoing (ExTINGUISH; NCT04372615) that investigates inebilizumab in anti-NMDAR-AIE [158]. Currently, no other trials are assessing inebilizumab in AIE.
5.4.1.4 Daratumumab (Anti-CD38 Antibody)
Daratumumab is a humanised anti-CD38 monoclonal antibody that is approved for the treatment of multiple myeloma. The CD38 epitope, an ADP-ribose hydroxylase, is upregulated in activated plasma cells. Treatment with daratumumab induces the death of these cells via various mechanisms, amongst others via complement-dependent cytotoxicity (CDC) [159]. In a study by Scheibe et al., five treatment-refractory AIE patients were included and daratumumab was administered in 4-20 cycles of 16 mg/kg which led to the clinical improvement of all patients. Moreover, it induced a significant reduction of autoantibodies in the serum and CSF suggesting daratumumab as a potential treatment option in otherwise non-responsive autoantibody-mediated AIE [160].
5.4.2 Agents Targeting Pathogenic Antibodies/Plasma Cells and Downstream Mechanisms
5.4.2.1 Rozanolixizumab, Efgartigimod-α (Blockade of FcRn)
The neonatal Fc receptor (FcRn) is a protein on endothelial cells that counteracts IgG degradation and extends IgG half-life by recycling and transcytosis of IgG within endothelial cells. Therefore, antagonism of FcRn increases IgG degradation leading to lower circulating levels of IgG (including pathogenic IgG autoantibodies) and more IgG degradation [161]. The neonatal Fc receptor is upregulated by proinflammatory cytokines. Emerging therapies use antibody fragments to target the FcRn, with the advantage of no widespread immunosuppression as in therapies with IVIG or PLEX [161, 162]. Rozanolixizumab is a humanised monoclonal anti-FcRn receptor IgG4 antibody. It binds the FcRn and therefore hinders recycling of IgG from the blood and leads to a massive decrease of circulating IgG [163]. There have been Phase 2 trials using rozanolixizumab in refractory acetylcholine receptor antibody-positive myasthenia gravis [164] and immune-thrombocytopenia [165]. There is currently one study investigating rozanolixizumab in anti-LGI1-AIE (NCT04875975)[166].
Efgartigimod-α is a humanised monoclonal antibody fragment that has the same mechanism of action as rozanolixizumab and is already used for refractory acetylcholine receptor antibody-positive myasthenia gravis [167]; however, no reports of use in AIE exist so far.
5.4.2.2 Bortezomib (Proteasome Inhibitor)
Bortezomib is a proteasome inhibitor that promotes apoptosis in plasma cells as was shown in SLE [168, 169]. It is successfully used for the treatment of multiple myeloma [170]. In AIE, Shin et al. investigated the subcutaneous administration of bortezomib in five patients with severe first- and second-line-, as well as tocilizumab-refractory anti-NMDAR-AIE. Clinical improvement was limited and hardly distinguishable from the natural course of disease [171]. However, clinical improvement or even disease remission, as well as the reduction of antibody titres upon administration of bortezomib has been reported in two different studies of severe therapy refractory anti-NMDAR-AIE [172, 173]. In a case report of a young woman with anti-NMDAR-AIE, bortezomib was administered within 42 days of hospitalisation after the patient had already received intravenous corticosteroids, IVIG, PLEX and RTX and did not improve clinically. The patient showed dramatic improvement within 15 days of bortezomib administration and was released from ICU [174]. Whether the effect still was due to bortezomib, a delayed effect of co-administered therapeutic agents, or the natural course of disease remains to be determined. Overall, bortezomib might represent a promising escalation therapy in severe cases of therapy-refractory AIE, although it has only been investigated in small cohorts.
Theoretically, and similar to daratumumab, a combination of B cell- and plasma cell- targeting therapies in refractory AIE should lead to a complete suppression of antibody production. A systematic review by Dinoto et al. [175], undertook a screening of PubMed literature, employing “Anti-N-Methyl-d-Aspartate encephalitis AND bortezomib” as search terms and identified 29 patients from 11 articles with anti-NMDAR-AIE who had received bortezomib as third-line therapy. All patients were treated with a CD20-depleting agent (RTX) beforehand and bortezomib was often administered late in the disease course. More than half of all patients (55%) had a favourable outcome; however, side effects, ranging from haematological, infectious to gastrointestinal, were reported in over one-third of all patients.
5.4.2.3 Eculizumab (Blocking the Complement Cascade)
The activation of the complement cascade has been discussed to play a significant role in the pathophysiology of AIE. Eculizumab is a humanised monoclonal anti-C5 complement protein-directed antibody, which acts by binding complement 5 and prevents cleavage of C5 into C5a and C5b by convertase [176]. It is administered in anti-MuSK-antibody-associated or otherwise drug refractory myasthenia gravis [177,178,179] and could be considered as a potential novel immunotherapeutic target in AIE with evidence for complement-mediated pathogenesis [180,181,182].
5.4.3 Agents Targeting or Modifying Cytokines
5.4.3.1 Tocilizumab and Satralizumab (IL-6 Blockers)
Tocilizumab and satralizumab are humanized monoclonal antibodies that are directed against the IL-6 receptor. By suppressing IL-6, differentiation of B cells into antibody-producing plasma cells (possibly even into long-lived plasma cells) driving the production of pathogenic autoantibodies, is reduced [183,184,185]. Tocilizumab has been administered in AIE patients refractory to RTX treatment, showing a favourable clinical response (improvement of modified Rankin Scale scores ≥ 2 at 2 months) compared to other immunotherapy strategies, as well as maintaining a long-term favourable clinical outcome [186]. Studies have demonstrated the successful use of tocilizumab as a second-line therapy notably in anti-Caspr2 AIE, suggesting that IL-6 activation might play a significant role in this type of AIE [187, 188]. One study in 2016 by Lee et al. [186] investigated patients with AIE who did not respond to first-line therapy and subsequent treatment with RTX. They divided the patients into three groups: (1) the first group was administered tocilizumab, (2) the second group continued RTX and (3) the third group was observational. Outcomes were assessed after 1 to at least 9 months after therapy and suggested best outcomes in patients receiving tocilizumab [186].
Another study by Lee et al. [189] in 2021 included 78 patients with paraneoplastic anti-NMDAR-AIE and teratoma who received the T-SIRT regime (i.e., Teratoma Removal, Steroids, IVIG, RTX and Tocilizumab). Including tocilizumab in the treatment regime lowered Clinical Assessment Scale for Autoimmune Encephalitis (CASE) scores (collected every 2 weeks for 12 weeks from baseline, every month for the next 3 months and then every 3 months) when compared to corticosteroids, IVIG and/or RTX only. They found that independent of AIE severity, rapid initiation of treatment yielded better outcome (completion of the SIRT regime within 1 month of onset resulted in better 1-year improvements in CASE scores and modified Rankin scale). However, these findings were only observed in patients exclusively with anti-NMDAR antibody-mediated- and teratoma-associated AIE. Larger study populations are needed to confirm these findings and to assess whether tocilizumab could emerge as a preferred second-line therapy in certain types of AIE. Moreover, there is also evidence that tocilizumab may be effective in paediatric cases; however, data is limited [190].
5.4.3.2 Anakinra and Canakinumab (IL-1 Blockers)
Anakinra is a synthetic analogue of the naturally occurring IL-1 receptor antagonist (IL-1Ra) and blocks the function of proinflammatory IL-1 [191] by blocking the IL-1 receptor (IL-1R). The effect of anakinra has shown effective prevention in the development of epilepsy following inflammation-associated epileptic seizures in animal models [192, 193]. One case involving a patient suffering from super refractory status epilepticus in AIE reported a reasonable recovery upon treatment with anakinra [194]. However, no clinical studies in AIE exist to date.
Canakinumab is a human monoclonal antibody directed against IL-1ß and is amongst others used to treat juvenile idiopathic arthritis [195] and refractory gout [196]. The use of canakinumab in AIE may be hampered by its failure to cross the intact BBB [197]; however, the BBB is likely to become more permissible in AIE and, thus, canakinumab may penetrate the brain parenchyma, as data showing that both canakinumab and anakinra can reach the brain compartment in significant concentration in an in vitro model [198]. A case report on a patient with generalised epilepsy and poor response to multiple ASMs-reported clinical response after therapeutic IL-1 blockade, first by treatment with anakinra followed by canakinumab with near resolution of seizures [199]. Additionally, a recent systematic review by Costagliola et al. showed that the use of anakinra/canakinumab reduced or arrested seizure frequency in over 50% of all patients [200].
5.4.3.3 Tumour Necrosis Factor-Alpha (TNFα Blocker)
Different types of IgG1 antibodies blocking the TNF-α receptor (such as adalimumab, certolizumab, infliximab, golimumab) are used for the treatment of various autoimmune diseases. However, anti-TNF-α antibodies have been also been associated with the exacerbation of autoimmune diseases, in particular multiple sclerosis [201]. There have been very few case reports investigating infliximab in AIE and only one report proposed infliximab as a potential treatment option [202]. Conversely, some patients developed AIE after the initiation of infliximab treatment [203]. A recent systematic review by Costagliola et al., highlighted a study by Lagarde et al. on the use of adalimumab in RE where a decrease in seizure frequency/seizure remission in more than 50% of patients was observed [145, 200].
5.4.3.4 Tofacitinib (JAK Inhibitor)
Tofacitinib is a Janus kinase inhibitor (JAK1 and JAK3) leading to the inhibition of signal transduction in cytokines [204]. It passes well through the BBB and is currently used in refractory rheumatoid and psoriasis arthritis. A case series by Jang et al. presented eight patients with AIE who received at least one of the following drugs: corticosteroids, IVIG, RTX or tocilizumab without showing clinical improvement. Two of the eight patients showed good response, three demonstrated partial response and three did not show clinical improvement (measured via clinical scores) upon tofacitinib administration. Except for mild nausea and transient neutropenia without fever in two patients, no adverse events or signs of acute toxicity were reported [205].
5.4.3.5 Low-Dose Interleukin-2 Therapy
In other cases, the function of IL-2 can be promoted, as an example: IL-2 activates T regulatory lymphocytes, which can attenuate autoimmune processes by restricting T effector cells [206]. Lim et al. investigated the effect of a low-dose IL-2 therapy in patients with AIE and resistance to first- and second-line immunotherapies. They found that six of ten patients showed an improvement in the modified Rankin scale, one patient experienced grade 3 serious adverse event suggesting that IL-2 might be a treatment option in therapy-refractory patients [207].
5.4.4 Other agents
5.4.4.1 Abatacept (CD80 and CD86 Ligand)
Abatacept is a humanised fusion protein consisting of a human IgG1 Fc region protein and the extracellular domain of cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), a protein receptor that functions as an immune checkpoint and is used in rheumatoid arthritis. By binding CD80 and CD86, abatacept blocks the activation of T cells. To date, one case of a child with juvenile idiopathic arthritis who was treated with abatacept developed anti-NMDAR AIE; however, the exact contribution of abatacept to the development of AIE is unknown [208].
5.4.4.2 Belnacasan (VX-765)
Belnacasan is a potent inhibitor of caspase-1. This enzyme is involved in inflammatory processes and mechanisms associated with pyro- and apoptosis; it also catalyses pro-interleukin-1 into its active form Il-1β [209]. Inhibition of the enzyme is likely to reduce the burden of inflammation, but was also suspected to reduce epileptic seizures in patients with chronic pharmaco-resistant focal epilepsy because of the inflammatory processes associated with chronic epilepsy. After convincing animal data [210], a Phase IIb study in pharmaco-resistant epilepsy patients (NCT01501383) was begun to evaluate safety and efficacy of this drug. The study was halted after enrolling 40 of the 60 planned patients by the company without official explanation, but there were no safety concerns [211]. After this drawback, the drug is currently in (pre-)clinical evaluation for cardio- and neuroprotection, and Alzheimer’s disease [212,213,214].
5.4.5 Therapeutic Drug Monitoring
A study by Syversen et al. [215] investigated the outcome of therapeutic drug monitoring (TDM) versus standard therapy in patients with diagnoses of rheumatoid arthritis, spondylarthritis, psoriatic arthritis, ulcerative colitis, Crohn’s disease and psoriasis receiving infliximab. Therapeutic drug monitoring meant that at each infusion serum levels of infliximab and antidrug antibodies were measured and results were used to guide dosage increase or decrease to reach the predetermined therapeutic range. Standard therapy meant administration of infliximab based on clinical judgement. Patients with TDM had significantly higher sustained disease control and an absence of disease worsening in follow-up of 52 weeks than patients with standard therapy (74% of patients in TDM group vs. 56% in standard group). While to date no studies investigating TDM in AIE exist, TDM in AIE could potentially be a promising approach to better sustain disease control.
5.5 Other Treatment Options in Drug-Resistant Epilepsy
5.5.1 Ketogenic Diet, Dysbiosis in Gut Microbiota and Probiotics in Drug-Resistant Epilepsy
Ketogenic diet is considered as an additional treatment option in epilepsy that is not responding to classic ASM, e.g., after two failed ASM treatments [216] and it almost completely abolishes seizure activity in a rare genetic epilepsy syndrome, glucose transporter-1 deficiency, within days to weeks [217]. In other syndromes, ketogenic diet can substantially decrease seizure frequency [218]. The ketogenic diet metabolism shifts the use of glucose to the use of fatty acids for energy, which results in the development of ketone bodies. Ketone bodies are thought to increase the seizure threshold by opening ATP-sensitive potassium channels and therefore results in neuronal membrane hyperpolarisation [219]. Many other mechanisms on a cellular, receptor or neurotransmitter level, like an increase in synaptic and whole brain GABA, may play additional roles in the antiseizure effect of the ketogenic diet [220, 221].
In that regard, it was shown that mice on a ketogenic diet were protected from electronically induced seizures [222]. Another study showed that gut microbiota were altered in patients with drug-resistant epilepsy with them yielding an increase in numerous rare bacteria. Interestingly, gut microbiota of epilepsy patients that responded to ASM was similar to healthy controls [223]. In a clinical trial by Gomez-Eguilaz et al. [224], patients with drug-resistant epilepsy underwent probiotic treatment administered for 4 months. Nearly one-third of all patients showed a > 50% reduction in number of seizures.
These findings point to changes in gut microbiota as a possible disease driving mechanism in drug-resistant epilepsy. Restoration of gut microbiota, for example by faecal transplantation or by administration of probiotics, could potentially serve as a new therapeutical approach.
5.5.2 Epilepsy Surgery
If pharmacological options are exhausted and epilepsy remains treatment resistant, surgical procedures such as resection of tissue with epileptogenic potential or the implantation of a neuromodulatory device can be evaluated. In a study by Li et al. [109], 86 patients with drug-resistant focal seizures were tested for autoantibodies before surgical intervention, 33% patients showed unspecific serum autoantibodies and 8% showed CNS-specific autoantibodies. These patients were potentially missed as patients with an epilepsy of autoimmune aetiology. Further diagnostic work-up specifically for underlying immunological aetiologies based on the APES score, and, if applicable, other treatment options such as immunomodulatory treatment should be tried before moving to surgical intervention. Even more so as the recent study of Carreno et al. shows that the outcome of epilepsy surgery in patients with drug-resistant temporal lobe epilepsy and presence of neuronal antibodies seems to be worse than in other patients without neuronal antibodies [225].
6 Conclusion and Perspective
There have been substantial advances in our pathophysiological understanding of immune-mediated seizures and epileptic syndromes in the past years. Yet, navigating through various clinical syndromes, namely AAE, AIE and ASSAE, can still pose major challenges in the clinical routine, which is due to (1) the clinical heterogeneous presentation with overlapping clinical phenotypes, and (2) the underdefined and changing terminology resulting in a lack of clear distinction between different disease entities. Unfortunately, the latter often results in the wrongful use of the term epilepsy in acute seizures of immune-mediated cause, which often subside when immune-mediated pathogenesis is correctly identified and treated. Missing the recognition of the immune-mediated aetiology not only results in incorrect treatment, but also in the wrongful diagnosis of epilepsy. This represents a major impact on patients’ lives by social restrictions such as driving. It remains a key challenge for physicians to clearly differentiate between ASSAE and AAE and to identify patients with epilepsy with an immune-mediated cause. However, at what timepoint enduring acute seizures are considered to have progressed to epilepsy remains a key question to be answered. Further, whether the higher frequency of neuronal autoantibodies in patients with epilepsy points to a pathogenic role of these antibodies or whether they are a bystander phenomenon raises an additional challenge. Here, the development of diagnostic scores to determine which patients with epilepsy to test for neuronal autoantibodies and subsequently to treat with immunotherapy has marked a major advancement in clinical practice. Rapid initiation of immunomodulation in immune-mediated seizures is crucial as ASMs often fail and are usually only applied add-on for symptomatic control of seizures.
To this date, no specific immunosuppressive therapy targeting the specific underlying antibody exists. Yet, novel immunotherapeutic strategies are emerging, targeting specific immune players of the involved immune cascade. Several trials are being conducted implementing these drugs in AIE. Moreover, new exciting clinical approaches such as therapeutic drug monitoring to attain better disease control are gaining attention. In the past years, dysbiosis and changes in gut microbiota have further been investigated in patients with therapy-refractory epilepsy, which opens up a completely novel field for clinical research with great therapeutic potential.
To conclude, seizures with an underlying immune-mediated aetiology are common and although the rapid and accurate identification holds many challenges for clinicians, immunomodulatory treatment often shows promising effects for patients. Awareness that seizures of immune aetiology of different aetiology and pathomechanisms exist is of high clinical relevance. Whilst pathomechanisms are investigated every day, we will surely learn more about novel immunotherapeutic strategies in the coming years.
References
Moshé SL, Perucca E, Ryvlin P, Tomson T. Epilepsy: new advances. Lancet. 2015;385(9971):884–98.
Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393(10172):689–701.
Beghi E. The epidemiology of epilepsy. Neuroepidemiology. 2020;54(2):185–91.
Steriade C, Gillinder L, Rickett K, Hartel G, Higdon L, Britton J, et al. Discerning the role of autoimmunity and autoantibodies in epilepsy: a review. JAMA Neurol. 2021;78(11):1383–90.
Dubey D, Alqallaf A, Hays R, Freeman M, Chen K, Ding K, et al. Neurological autoantibody prevalence in epilepsy of unknown etiology. JAMA Neurol. 2017;74(4):397–402.
Elisak M, Krysl D, Hanzalova J, Volna K, Bien CG, Leypoldt F, et al. The prevalence of neural antibodies in temporal lobe epilepsy and the clinical characteristics of seropositive patients. Seizure. 2018;63:1–6.
Nóbrega-Jr AW, Gregory CP, Schlindwein-Zanini R, Neves FS, Wolf P, Walz R, et al. Mesial temporal lobe epilepsy with hippocampal sclerosis is infrequently associated with neuronal autoantibodies. Epilepsia. 2018;59(9):e152–6.
de Bruijn M, Bastiaansen AEM, Mojzisova H, van Sonderen A, Thijs RD, Majoie MJM, et al. Antibodies contributing to focal epilepsy signs and symptoms score. Ann Neurol. 2021;89(4):698–710.
Lancaster E. The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol (Seoul, Korea). 2016;12(1):1–13.
Geis C, Planagumà J, Carreño M, Graus F, Dalmau J. Autoimmune seizures and epilepsy. J Clin Invest. 2019;129(3):926–40.
Dalmau J, Geis C, Graus F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev. 2017;97(2):839–87.
Beghi E, Carpio A, Forsgren L, Hesdorffer DC, Malmgren K, Sander JW, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51(4):671–5.
Steriade C, Britton J, Dale RC, Gadoth A, Irani SR, Linnoila J, et al. Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune-associated epilepsy: conceptual definitions. Epilepsia. 2020;61(7):1341–51.
Abboud H, Probasco JC, Irani S, Ances B, Benavides DR, Bradshaw M, et al. Autoimmune encephalitis: proposed best practice recommendations for diagnosis and acute management. J Neurol Neurosurg Psychiatry. 2021;92(7):757–68.
Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404.
Fisher RS, van Emde BW, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46(4):470–2.
Spatola M, Dalmau J. Seizures and risk of epilepsy in autoimmune and other inflammatory encephalitis. Curr Opin Neurol. 2017;30(3):345–53.
Yeshokumar AK, Coughlin A, Fastman J, Psaila K, Harmon M, Randell T, et al. Seizures in autoimmune encephalitis-A systematic review and quantitative synthesis. Epilepsia. 2021;62(2):397–407.
Ilyas-Feldmann M, Prüß H, Holtkamp M. Long-term seizure outcome and antiseizure medication use in autoimmune encephalitis. Seizure. 2021;86:138–43.
Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–21.
Rüegg SJ, Jungi TW. Antibody-mediated erythrolysis and erythrophagocytosis by human monocytes, macrophages and activated macrophages. Evidence for distinction between involvement of high-affinity and low-affinity receptors for IgG by using different erythroid target cells. Immunology. 1988;63(3):513–20.
Dalakas MC. B cells in the pathophysiology of autoimmune neurological disorders: a credible therapeutic target. Pharmacol Ther. 2006;112(1):57–70.
Ehling P, Melzer N, Budde T, Meuth SG. CD8+ T cell-mediated neuronal dysfunction and degeneration in limbic encephalitis. Front Neurol. 2015;15(6):153.
Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835–44.
Graus F, Saiz A, Dalmau J. Antibodies and neuronal autoimmune disorders of the CNS. J Neurol. 2010;257(4):509–17.
Newman M, Airey C, Blum S, Scott JG, Wong RC, Gillis D. Chapter 9—autoimmune encephalitis: clinical features, pathophysiology, and management. In: Minagar A, editor. Neuroinflammation (Second Edition): Academic Press; 2018. pp. 193-216.
Rosenfeld MR, Titulaer MJ, Dalmau J. Paraneoplastic syndromes and autoimmune encephalitis: five new things. Neurol Clin Pract. 2012;2(3):215–23.
Huijbers MG, Plomp JJ, van der Maarel SM, Verschuuren JJ. IgG4-mediated autoimmune diseases: a niche of antibody-mediated disorders. Ann N Y Acad Sci. 2018;1413(1):92–103.
Saitakis G, Chwalisz BK. The neurology of IGG4-related disease. J Neurol Sci. 2021;424: 117420.
Blinder T, Lewerenz J. Cerebrospinal fluid findings in patients with autoimmune encephalitis-a systematic analysis. Front Neurol. 2019;10:804.
van Sonderen A, Ariño H, Petit-Pedrol M, Leypoldt F, Körtvélyessy P, Wandinger K-P, et al. The clinical spectrum of Caspr2 antibody-associated disease. Neurology. 2016;87(5):521–8.
Dalakas MC, Alexopoulos H, Spaeth PJ. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat Rev Neurol. 2020;16(11):601–17.
Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30(17):5866–75.
Flanagan EP, Kotsenas AL, Britton JW, McKeon A, Watson RE, Klein CJ, et al. Basal ganglia T1 hyperintensity in LGI1-autoantibody faciobrachial dystonic seizures. Neurol Neuroimmunol Neuroinflamm. 2015;2(6): e161.
Heine J, Prüss H, Kopp UA, Wegner F, Then Bergh F, Münte T, et al. Beyond the limbic system: disruption and functional compensation of large-scale brain networks in patients with anti-LGI1 encephalitis. J Neurol Neurosurg Psychiatry. 2018;89(11):1191–9.
Chabrol E, Navarro V, Provenzano G, Cohen I, Dinocourt C, Rivaud-Péchoux S, et al. Electroclinical characterization of epileptic seizures in leucine-rich, glioma-inactivated 1-deficient mice. Brain. 2010;133(9):2749–62.
Ramberger M, Berretta A, Tan JMM, Sun B, Michael S, Yeo T, et al. Distinctive binding properties of human monoclonal LGI1 autoantibodies determine pathogenic mechanisms. Brain. 2020;143(6):1731–45.
Kornau HC, Kreye J, Stumpf A, Fukata Y, Parthier D, Sammons RP, et al. Human cerebrospinal fluid monoclonal LGI1 autoantibodies increase neuronal excitability. Ann Neurol. 2020;87(3):405–18.
Baudin P, Whitmarsh S, Cousyn L, Roussel D, Lecas S, Lehongre K, et al. Kv1.1 channels inhibition in the rat motor cortex recapitulates seizures associated with anti-LGI1 encephalitis. Prog Neurobiol. 2022;213:102262.
Petit-Pedrol M, Sell J, Planagumà J, Mannara F, Radosevic M, Haselmann H, et al. LGI1 antibodies alter Kv1.1 and AMPA receptors changing synaptic excitability, plasticity and memory. Brain. 2018;141(11):3144–59.
Peng X, Hughes EG, Moscato EH, Parsons TD, Dalmau J, Balice-Gordon RJ. Cellular plasticity induced by anti-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor encephalitis antibodies. Ann Neurol. 2015;77(3):381–98.
Ohkawa T, Satake S, Yokoi N, Miyazaki Y, Ohshita T, Sobue G, et al. Identification and characterization of GABA(A) receptor autoantibodies in autoimmune encephalitis. J Neurosci. 2014;34(24):8151–63.
Spatola M, Petit-Pedrol M, Simabukuro MM, Armangue T, Castro FJ, Barcelo Artigues MI, et al. Investigations in GABA(A) receptor antibody-associated encephalitis. Neurology. 2017;88(11):1012–20.
Noviello CM, Kreye J, Teng J, Prüss H, Hibbs RE. Structural mechanisms of GABA(A) receptor autoimmune encephalitis. Cell. 2022;185(14):2469-77.e13.
Duong SL, Prüss H. Molecular disease mechanisms of human antineuronal monoclonal autoantibodies. Trends Mol Med. 2023; 29(1): 20-34.
Nibber A, Mann EO, Pettingill P, Waters P, Irani SR, Kullmann DM, et al. Pathogenic potential of antibodies to the GABA(B) receptor. Epilepsia Open. 2017;2(3):355–9.
Ishida K, Mitoma H, Song SY, Uchihara T, Inaba A, Eguchi S, et al. Selective suppression of cerebellar GABAergic transmission by an autoantibody to glutamic acid decarboxylase. Ann Neurol. 1999;46(2):263–7.
Stagg CJ, Lang B, Best JG, McKnight K, Cavey A, Johansen-Berg H, et al. Autoantibodies to glutamic acid decarboxylase in patients with epilepsy are associated with low cortical GABA levels. Epilepsia. 2010;51(9):1898–901.
Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol. 2010;67(4):470–8.
Li X, Guo Q, Zheng Z, Wang X, Liu S. Immune-mediated epilepsy with GAD65 antibodies. J Neuroimmunol. 2020;15(341): 577189.
Kim J, Namchuk M, Bugawan T, Fu Q, Jaffe M, Shi Y, et al. Higher autoantibody levels and recognition of a linear NH2-terminal epitope in the autoantigen GAD65, distinguish stiff-man syndrome from insulin-dependent diabetes mellitus. J Exp Med. 1994;180(2):595–606.
Daw K, Ujihara N, Atkinson M, Powers AC. Glutamic acid decarboxylase autoantibodies in stiff-man syndrome and insulin-dependent diabetes mellitus exhibit similarities and differences in epitope recognition. J Immunol. 1996;156(2):818–25.
Leypoldt F, Titulaer MJ, Aguilar E, Walther J, Bönstrup M, Havemeister S, et al. Herpes simplex virus-1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology. 2013;81(18):1637–9.
DeSena A, Graves D, Warnack W, Greenberg BM. Herpes simplex encephalitis as a potential cause of anti–N-methyl-d-aspartate receptor antibody encephalitis: report of 2 cases. JAMA Neurol. 2014;71(3):344–6.
Armangue T, Spatola M, Vlagea A, Mattozzi S, Cárceles-Cordon M, Martinez-Heras E, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760–72.
Nosadini M, Mohammad SS, Corazza F, Ruga EM, Kothur K, Perilongo G, et al. Herpes simplex virus-induced anti-N-methyl-d-aspartate receptor encephalitis: a systematic literature review with analysis of 43 cases. Dev Med Child Neurol. 2017;59(8):796–805.
Alexopoulos H, Kouremenos E, Akrivou S, Naoumis D, Antonopoulou M, Vlachoyiannopoulos P, et al. Post-Herpes Simplex Virus (HSV) Autoimmune Encephalitis: A Case Series and Novel Immunological Findings (P1.292). Neurology. 2016;86(16 Supplement):P1.292.
Solís N, Salazar L, Hasbun R. Anti-NMDA receptor antibody encephalitis with concomitant detection of Varicella zoster virus. J Clin Virol. 2016;83:26–8.
Schäbitz W-R, Rogalewski A, Hagemeister C, Bien CG. VZV brainstem encephalitis triggers NMDA receptor immunoreaction. Neurology. 2014;83(24):2309–11.
Hou R, Wu J, He D, Yan Y, Li L. Anti-N-methyl-D-aspartate receptor encephalitis associated with reactivated Epstein-Barr virus infection in pediatric patients: three case reports. Med (Baltim). 2019;98(20): e15726.
Valencia Sanchez C, Theel E, Binnicker M, Toledano M, McKeon A. Autoimmune encephalitis after SARS-CoV-2 infection. Case frequency, findings, and outcomes. Neurology 2021;97(23):e2262-e8.
Asadi-Pooya AA. Seizures associated with coronavirus infections. Seizure. 2020;79:49–52.
Carroll E, Melmed KR, Frontera J, Placantonakis DG, Galetta S, Balcer L, et al. Cerebrospinal fluid findings in patients with seizure in the setting of COVID-19: a review of the literature. Seizure. 2021;89:99–106.
van Sonderen A, Roelen DL, Stoop JA, Verdijk RM, Haasnoot GW, Thijs RD, et al. Anti-LGI1 encephalitis is strongly associated with HLA-DR7 and HLA-DRB4. Ann Neurol. 2017;81(2):193–8.
Mueller SH, Färber A, Prüss H, Melzer N, Golombeck KS, Kümpfel T, et al. Genetic predisposition in anti-LGI1 and anti-NMDA receptor encephalitis. Ann Neurol. 2018;83(4):863–9.
Strippel C, Herrera-Rivero M, Wendorff M, Tietz AK, Degenhardt F, Witten A, et al. A genome-wide association study in autoimmune neurological syndromes with anti-GAD65 autoantibodies. Brain. 2022; Mar 28:awac119.https://doi.org/10.1093/brain/awac119. Online ahead of print. PMID: 35348614
Binks S, Varley J, Lee W, Makuch M, Elliott K, Gelfand JM, et al. Distinct HLA associations of LGI1 and CASPR2-antibody diseases. Brain. 2018;141(8):2263–71.
Kim TJ, Lee ST, Moon J, Sunwoo JS, Byun JI, Lim JA, et al. Anti-LGI1 encephalitis is associated with unique HLA subtypes. Ann Neurol. 2017;81(2):183–92.
van Vliet EA, Aronica E, Gorter JA. Blood-brain barrier dysfunction, seizures and epilepsy. Semin Cell Dev Biol. 2015;38:26–34.
Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med. 2018;378(9):840–51.
Lin Z, Si Q, Xiaoyi Z. Association between epilepsy and systemic autoimmune diseases: a meta-analysis. Seizure. 2016;41:160–6.
Ong MS, Kohane IS, Cai T, Gorman MP, Mandl KD. Population-level evidence for an autoimmune etiology of epilepsy. JAMA Neurol. 2014;71(5):569–74.
Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7(1):31–40.
Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 2015;96:70–82.
Vezzani A, Balosso S, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. 2019;15(8):459–72.
Youn Y, Sung IK, Lee IG. The role of cytokines in seizures: interleukin (IL)-1β, IL-1Ra, IL-8, and IL-10. Korean J Pediatr. 2013;56(7):271–4.
Zhou C, Qi C, Zhao J, Wang F, Zhang W, Li C, et al. Interleukin-1β inhibits voltage-gated sodium currents in a time- and dose-dependent manner in cortical neurons. Neurochem Res. 2011;36(6):1116–23.
Ruffolo G, Alfano V, Romagnolo A, Zimmer T, Mills JD, Cifelli P, et al. GABA(A) receptor function is enhanced by Interleukin-10 in human epileptogenic gangliogliomas and its effect is counteracted by Interleukin-1β. Sci Rep. 2022;12(1):17956.
Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4(7):702–10.
Terrone G, Pauletti A, Salamone A, Rizzi M, Villa BR, Porcu L, et al. Inhibition of monoacylglycerol lipase terminates diazepam-resistant status epilepticus in mice and its effects are potentiated by a ketogenic diet. Epilepsia. 2018;59(1):79–91.
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–40.
Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L. The role of oxidative stress during inflammatory processes. Biol Chem. 2014;395(2):203–30.
Devinsky O, Schein A, Najjar S. Epilepsy associated with systemic autoimmune disorders. Epilepsy Curr. 2013;13(2):62–8.
Daneman R. The blood-brain barrier in health and disease. Ann Neurol. 2012;72(5):648–72.
Janigro D. Are you in or out? Leukocyte, ion, and neurotransmitter permeability across the epileptic blood-brain barrier. Epilepsia. 2012;53(Suppl 1):26–34.
Salar S, Maslarova A, Lippmann K, Nichtweiss J, Weissberg I, Sheintuch L, et al. Blood-brain barrier dysfunction can contribute to pharmacoresistance of seizures. Epilepsia. 2014;55(8):1255–63.
Bar-Klein G, Lublinsky S, Kamintsky L, Noyman I, Veksler R, Dalipaj H, et al. Imaging blood-brain barrier dysfunction as a biomarker for epileptogenesis. Brain. 2017;140(6):1692–705.
Weissberg I, Wood L, Kamintsky L, Vazquez O, Milikovsky DZ, Alexander A, et al. Albumin induces excitatory synaptogenesis through astrocytic TGF-β/ALK5 signaling in a model of acquired epilepsy following blood-brain barrier dysfunction. Neurobiol Dis. 2015;78:115–25.
Löscher W, Friedman A. Structural, molecular, and functional alterations of the blood-brain barrier during epileptogenesis and epilepsy: a cause, consequence, or both? Int J Mol Sci. 2020;21:2.
Frigerio F, Frasca A, Weissberg I, Parrella S, Friedman A, Vezzani A, et al. Long-lasting pro-ictogenic effects induced in vivo by rat brain exposure to serum albumin in the absence of concomitant pathology. Epilepsia. 2012;53(11):1887–97.
Varadkar S, Bien CG, Kruse CA, Jensen FE, Bauer J, Pardo CA, et al. Rasmussen’s encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol. 2014;13(2):195–205.
Rogers SW, Andrews PI, Gahring LC, Whisenand T, Cauley K, Crain B, et al. Autoantibodies to glutamate receptor GluR3 in Rasmussen’s encephalitis. Science. 1994;265(5172):648–51.
Watson R, Jiang Y, Bermudez I, Houlihan L, Clover L, McKnight K, et al. Absence of antibodies to glutamate receptor type 3 (GluR3) in Rasmussen encephalitis. Neurology. 2004;63(1):43–50.
Orsini A, Foiadelli T, Carli N, Costagliola G, Masini B, Bonuccelli A, et al. Rasmussen’s encephalitis: from immune pathogenesis towards targeted-therapy. Seizure. 2020;81:76–83.
Ramaswamy V, Walsh JG, Sinclair DB, Johnson E, Tang-Wai R, Wheatley BM, et al. Inflammasome induction in Rasmussen’s encephalitis: cortical and associated white matter pathogenesis. J Neuroinflamm. 2013;10(1):918.
Tröscher AR, Wimmer I, Quemada-Garrido L, Köck U, Gessl D, Verberk SGS, et al. Microglial nodules provide the environment for pathogenic T cells in human encephalitis. Acta Neuropathol. 2019;137(4):619–35.
Tang C, Yang W, Luan G. Progress in pathogenesis and therapy of Rasmussen’s encephalitis. Acta Neurol Scand. 2022;146(6):761–6.
Tang C, Wang X, Deng J, Xiong Z, Guan Y, Zhou J, et al. Increased inflammasome-activated pyroptosis mediated by caspase-1 in Rasmussen’s encephalitis. Epilepsy Res. 2021;18(179): 106843.
Leitner DF, Lin Z, Sawaged Z, Kanshin E, Friedman D, Devore S, et al. Brain Molecular Mechanisms in Rasmussen Encephalitis. Epilepsia. 2022. https://doi.org/10.1111/epi.17457.
Dubey D, Blackburn K, Greenberg B, Stuve O, Vernino S. Diagnostic and therapeutic strategies for management of autoimmune encephalopathies. Expert Rev Neurother. 2016;16(8):937–49.
Chen B, Lopez Chiriboga AS, Sirven JI, Feyissa AM. Autoimmune encephalitis-related seizures and epilepsy: diagnostic and therapeutic approaches. Mayo Clin Proc. 2021;96(8):2029–39.
Irani SR, Michell AW, Lang B, Pettingill P, Waters P, Johnson MR, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69(5):892–900.
Varley JA, Webb AJS, Balint B, Fung VSC, Sethi KD, Tijssen MAJ, et al. The Movement disorder associated with NMDAR antibody-encephalitis is complex and characteristic: an expert video-rating study. J Neurol Neurosurg Psychiatry. 2019;90(6):724–6.
Petit-Pedrol M, Armangue T, Peng X, Bataller L, Cellucci T, Davis R, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13(3):276–86.
Leypoldt F, Wandinger KP, Bien CG, Dalmau J. Autoimmune encephalitis. Eur Neurol Rev. 2013;8(1):31–7.
Steriade C, Moosa ANV, Hantus S, Prayson RA, Alexopoulos A, Rae-Grant A. Electroclinical features of seizures associated with autoimmune encephalitis. Seizure. 2018;60:198–204.
De Leiris N, Ruel B, Vervandier J, Boucraut J, Grimaldi S, Horowitz T, et al. Decrease in the cortex/striatum metabolic ratio on [(18)F]-FDG PET: a biomarker of autoimmune encephalitis. Eur J Nucl Med Mol Imaging. 2022;49(3):921–31.
Dubey D, Pittock SJ, Kelly CR, McKeon A, Lopez-Chiriboga AS, Lennon VA, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83(1):166–77.
Li Y, Tymchuk S, Barry J, Muppidi S, Le S. Antibody prevalence in epilepsy before surgery (APES) in drug-resistant focal epilepsy. Epilepsia. 2021;62(3):720–8.
Rada A, Birnbacher R, Gobbi C, Kurthen M, Ludolph A, Naumann M, et al. Seizures associated with antibodies against cell surface antigens are acute symptomatic and not indicative of epilepsy: insights from long-term data. J Neurol. 2021;268(3):1059–69.
Chen SS, Zhang YF, Di Q, Shi JP, Wang LL, Lin XJ, et al. Predictors and prognoses of epilepsy after anti-neuronal antibody-positive autoimmune encephalitis. Seizure. 2021;92:189–94.
Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–65.
Bartolini L. Practice current: how do you treat anti-NMDA receptor encephalitis? Neurol Clin Pract. 2016;6(1):69–72.
Bartolini L, Muscal E. Differences in treatment of anti-NMDA receptor encephalitis: results of a worldwide survey. J Neurol. 2017;264(4):647–53.
Sechi E, Flanagan EP. Antibody-mediated autoimmune diseases of the CNS: challenges and approaches to diagnosis and management. Front Neurol. 2021;12: 673339.
Jiang Y, Tian X, Gu Y, Li F, Wang X. Application of plasma exchange in steroid-responsive encephalopathy. Front Immunol. 2019;10:324.
Heine J, Ly LT, Lieker I, Slowinski T, Finke C, Prüss H, et al. Immunoadsorption or plasma exchange in the treatment of autoimmune encephalitis: a pilot study. J Neurol. 2016;263(12):2395–402.
Dogan Onugoren M, Golombeck KS, Bien C, Abu-Tair M, Brand M, Bulla-Hellwig M, et al. Immunoadsorption therapy in autoimmune encephalitides. Neurol Neuroimmunol Neuroinflamm. 2016;3(2): e207.
Macher S, Zimprich F, De Simoni D, Höftberger R, Rommer PS. Management of autoimmune encephalitis: an observational monocentric study of 38 patients. Front Immunol. 2018;22:9.
de Bruijn MAAM, van Sonderen A, van Coevorden-Hameete MH, Bastiaansen AEM, Schreurs MWJ, Rouhl RPW, et al. Evaluation of seizure treatment in anti-LGI1, anti-NMDAR, and anti-GABA<sub>B</sub>R encephalitis. Neurology. 2019;92(19):e2185–96.
Cuttle L, Munns AJ, Hogg NA, Scott JR, Hooper WD, Dickinson RG, et al. Phenytoin metabolism by human cytochrome P450: involvement of P450 3A and 2C forms in secondary metabolism and drug-protein adduct formation. Drug Metab Dispos. 2000;28(8):945–50.
Jubiz W, Meikle AW, Levinson RA, Mizutani S, West CD, Tyler FH. Effect of diphenylhydantoin on the metabolism of dexamethasone. N Engl J Med. 1970;283(1):11–4.
Nation RL, Evans AM, Milne RW. Pharmacokinetic drug interactions with phenytoin (Part II). Clin Pharmacokinet. 1990;18(2):131–50.
Patsalos PN, Perucca E. Clinically important drug interactions in epilepsy: general features and interactions between antiepileptic drugs. Lancet Neurol. 2003;2(6):347–56.
Koehler PJ. Use of corticosteroids in neuro-oncology. Anticancer Drugs. 1995;6(1):19–33.
Audet-Walsh E, Auclair-Vincent S, Anderson A. Glucocorticoids and phenobarbital induce murine CYP2B genes by independent mechanisms. Expert Opin Drug Metab Toxicol. 2009;5(12):1501–11.
Wen X, Wang JS, Kivistö KT, Neuvonen PJ, Backman JT. In vitro evaluation of valproic acid as an inhibitor of human cytochrome P450 isoforms: preferential inhibition of cytochrome P450 2C9 (CYP2C9). Br J Clin Pharmacol. 2001;52(5):547–53.
Schmitt C, Kuhn B, Zhang X, Kivitz AJ, Grange S. Disease-drug-drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacol Ther. 2011;89(5):735–40.
Berman E, Noyman I, Medvedovsky M, Ekstein D, Eyal S. Not your usual drug-drug interactions: monoclonal antibody-based therapeutics may interact with antiseizure medications. Epilepsia. 2022;63(2):271–89.
Dalmau J, Rosenfeld MR. Autoimmune encephalitis update. Neuro Oncol. 2014;16(6):771–8.
Gabilondo I, Saiz A, Galán L, González V, Jadraque R, Sabater L, et al. Analysis of relapses in anti-NMDAR encephalitis. Neurology. 2011;77(10):996–9.
Abboud H, Probasco J, Irani SR, Ances B, Benavides DR, Bradshaw M, et al. Autoimmune encephalitis: proposed recommendations for symptomatic and long-term management. J Neurol Neurosurg Psychiatry. 2021;92(8):897–907.
Zuliani L, Nosadini M, Gastaldi M, Spatola M, Iorio R, Zoccarato M, et al. Management of antibody-mediated autoimmune encephalitis in adults and children: literature review and consensus-based practical recommendations. Neurol Sci. 2019;40(10):2017–30.
Stuby J, Herren T, Schwegler Naumburger G, Papet C, Rudiger A. Immune checkpoint inhibitor therapy-associated encephalitis: a case series and review of the literature. Swiss Med Wkly. 2020;16(150): w20377.
Dubey D, Singh J, Britton JW, Pittock SJ, Flanagan EP, Lennon VA, et al. Predictive models in the diagnosis and treatment of autoimmune epilepsy. Epilepsia. 2017;58(7):1181–9.
Dubey D, Kothapalli N, McKeon A, Flanagan EP, Lennon VA, Klein CJ, et al. Predictors of neural-specific autoantibodies and immunotherapy response in patients with cognitive dysfunction. J Neuroimmunol. 2018;323:62–72.
Balu R, McCracken L, Lancaster E, Graus F, Dalmau J, Titulaer MJ. A score that predicts 1-year functional status in patients with anti-NMDA receptor encephalitis. Neurology. 2019;92(3):e244–52.
Toledano M, Britton JW, McKeon A, Shin C, Lennon VA, Quek AM, et al. Utility of an immunotherapy trial in evaluating patients with presumed autoimmune epilepsy. Neurology. 2014;82(18):1578–86.
von Rhein B, Wagner J, Widman G, Malter MP, Elger CE, Helmstaedter C. Suspected antibody negative autoimmune limbic encephalitis: outcome of immunotherapy. Acta Neurol Scand. 2017;135(1):134–41.
Granata T, Fusco L, Gobbi G, Freri E, Ragona F, Broggi G, et al. Experience with immunomodulatory treatments in Rasmussen’s encephalitis. Neurology. 2003;61(12):1807–10.
Andrews PI, Dichter MA, Berkovic SF, Newton MR, McNamara JO. Plasmapheresis in Rasmussen’s encephalitis. Neurology. 1996;46(1):242–6.
Palace J, Lang B. Epilepsy: an autoimmune disease? J Neurol Neurosurg Psychiatry. 2000;69(6):711–4.
Bien CG, Tiemeier H, Sassen R, Kuczaty S, Urbach H, von Lehe M, et al. Rasmussen encephalitis: incidence and course under randomized therapy with tacrolimus or intravenous immunoglobulins. Epilepsia. 2013;54(3):543–50.
Pellegrin S, Baldeweg T, Pujar S, D’Arco F, Cantalupo G, Varadkar S, et al. Immunomodulation with azathioprine therapy in Rasmussen syndrome: a multimodal evaluation. Neurology. 2021;96(2):e267–79.
Lagarde S, Villeneuve N, Trébuchon A, Kaphan E, Lepine A, McGonigal A, et al. Anti-tumor necrosis factor alpha therapy (adalimumab) in Rasmussen’s encephalitis: An open pilot study. Epilepsia. 2016;57(6):956–66.
Cay-Martinez KC, Hickman RA, McKhann Ii GM, Provenzano FA, Sands TT. Rasmussen encephalitis: an update. Semin Neurol. 2020;40(2):201–10.
Hachiya Y, Uruha A, Kasai-Yoshida E, Shimoda K, Satoh-Shirai I, Kumada S, et al. Rituximab ameliorates anti-N-methyl-D-aspartate receptor encephalitis by removal of short-lived plasmablasts. J Neuroimmunol. 2013;265(1–2):128–30.
Mahévas M, Michel M, Weill J-C, Reynaud C-A. Long-lived plasma cells in autoimmunity: lessons from B-cell depleting therapy. Front Immunol. 2013;4:494.
Khosroshahi A, Bloch DB, Deshpande V, Stone JH. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum. 2010;62(6):1755–62.
Marino M, Basile U, Spagni G, Napodano C, Iorio R, Gulli F, et al. Long-lasting rituximab-induced reduction of specific-but not total-IgG4 in MuSK-positive myasthenia gravis. Front Immunol. 2020;11:613.
Lee DSW, Rojas OL, Gommerman JL. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat Rev Drug Discov. 2021;20(3):179–99.
Sacco KA, Abraham RS. Consequences of B-cell-depleting therapy: hypogammaglobulinemia and impaired B-cell reconstitution. Immunotherapy. 2018;10(8):713–28.
Filippini G, Kruja J, Del Giovane C. Rituximab for people with multiple sclerosis. Cochrane Database Syst Rev. 2021;11(11):CD013874.
Greenfield AL, Hauser SL. B-cell therapy for multiple sclerosis: entering an era. Ann Neurol. 2018;83(1):13–26.
Sorensen PS, Blinkenberg M. The potential role for ocrelizumab in the treatment of multiple sclerosis: current evidence and future prospects. Ther Adv Neurol Disord. 2016;9(1):44–52.
Blackburn KM, Denney DA, Hopkins SC, Vernino SA. Low recruitment in a double-blind, placebo-controlled trial of ocrelizumab for autoimmune encephalitis: a case series and review of lessons learned. Neurol Ther. 2022;11:893–903.
Cree BAC, Bennett JL, Kim HJ, Weinshenker BG, Pittock SJ, Wingerchuk DM, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394(10206):1352–63.
The ExTINGUISH Trial of Inebilizumab in NMDAR Encephalitis (ExTINGUISH). 2020. https://clinicaltrials.gov/ct2/show/NCT04372615. Accessed 4 May 2021.
de Weers M, Tai Y-T, van der Veer MS, Bakker JM, Vink T, Jacobs DCH, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–8.
Scheibe F, Ostendorf L, Prüss H, Radbruch H, Aschman T, Hoffmann S, et al. Daratumumab for treatment-refractory antibody-mediated diseases in neurology. Eur J Neurol. 2022;29(6):1847–54.
Gable KL, Guptill JT. Antagonism of the neonatal Fc receptor as an emerging treatment for myasthenia Gravis. Front Immunol. 2020;10:3052.
Nelke C, Spatola M, Schroeter CB, Wiendl H, Lünemann JD. Neonatal Fc receptor-targeted therapies in neurology. Neurotherapeutics. 2022;19(3):729–40.
Kiessling P, Lledo-Garcia R, Watanabe S, Langdon G, Tran D, Bari M, et al. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: a randomized phase 1 study. Sci Transl Med. 2017;9:414.
Bril V, Benatar M, Andersen H, Vissing J, Brock M, Greve B, et al. Efficacy and safety of rozanolixizumab in moderate to severe generalized myasthenia Gravis. A phase 2 randomized control trial. Neurology. 2021;96(6):e853–65.
Robak T, Kaźmierczak M, Jarque I, Musteata V, Treliński J, Cooper N, et al. Phase 2 multiple-dose study of an FcRn inhibitor, rozanolixizumab, in patients with primary immune thrombocytopenia. Blood Adv. 2020;4(17):4136–46.
SRL UB. A study to test the efficacy, safety, and pharmacokinetics of rozanolixizumab in adult study participants with leucine-rich glioma inactivated 1 autoimmune encephalitis. 2021. https://clinicaltrials.gov/ct2/show/NCT04372615. Accessed 13 Apr 2022.
Howard JF Jr, Bril V, Vu T, Karam C, Peric S, Margania T, et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021;20(7):526–36.
Neubert K, Meister S, Moser K, Weisel F, Maseda D, Amann K, et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14(7):748–55.
Alexander T, Sarfert R, Klotsche J, Kühl AA, Rubbert-Roth A, Lorenz HM, et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann Rheum Dis. 2015;74(7):1474–8.
Scott K, Hayden PJ, Will A, Wheatley K, Coyne I. Bortezomib for the treatment of multiple myeloma. Cochrane Database Syst Rev. 2016;20(4):CD010816.
Shin Y-W, Lee S-T, Kim T-J, Jun J-S, Chu K. Bortezomib treatment for severe refractory anti-NMDA receptor encephalitis. Ann Clin Transl Neurol. 2018;5(5):598–605.
Scheibe F, Prüss H, Mengel AM, Kohler S, Nümann A, Köhnlein M, et al. Bortezomib for treatment of therapy-refractory anti-NMDA receptor encephalitis. Neurology. 2017;88(4):366–70.
Keddie S, Crisp SJ, Blackaby J, Cox A, Coles A, Hart M, et al. Plasma cell depletion with bortezomib in the treatment of refractory N-methyl-d-aspartate (NMDA) receptor antibody encephalitis. Rational developments in neuroimmunological treatment. Eur J Neurol. 2018;25(11):1384–8.
Turnbull MT, Siegel JL, Becker TL, Stephens AJ, Lopez-Chiriboga AS, Freeman WD. Early bortezomib therapy for refractory anti-NMDA receptor encephalitis. Front Neurol. 2020;11:188.
Dinoto A, Cheli M, Bratina A, Sartori A, Manganotti P. Bortezomib in anti-N-Methyl-d-aspartate-receptor (NMDA-R) encephalitis: a systematic review. J Neuroimmunol. 2021;356: 577586.
Eisenberg R. Chapter 49—immune compromise associated with biologics. In: Sullivan KE, Stiehm ER, editors. Stiehm’s immune deficiencies. Amsterdam: Academic Press; 2014. p. 889–906.
Datta S, Singh S, Govindarajan R. Retrospective analysis of eculizumab in patients with acetylcholine receptor antibody-negative myasthenia gravis: a case series. J Neuromusc Dis. 2020;7(3):269–77.
Singh S, Singh H, Datta S, Govindarajan R. Eculizumab in the treatment of seronegative refractory generalized myasthenia gravis. Neurology. 2020;94(Suppl 15):1691.
Howard JF, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017;16(12):976–86.
Bien CG, Vincent A, Barnett MH, Becker AJ, Blümcke I, Graus F, et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain. 2012;135(Pt 5):1622–38.
Smets I, Titulaer MJ. Antibody therapies in autoimmune encephalitis. Neurotherapeutics. 2022;19(3):823–31.
Gill AJ, Venkatesan A. Pathogenic mechanisms in neuronal surface autoantibody-mediated encephalitis. J Neuroimmunol. 2022;15(368): 577867.
Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspec Biol. 2014;6(10): a016295.
Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171(4):1684–90.
Jourdan M, Cren M, Robert N, Bolloré K, Fest T, Duperray C, et al. IL-6 supports the generation of human long-lived plasma cells in combination with either APRIL or stromal cell-soluble factors. Leukemia. 2014;28(8):1647–56.
Lee WJ, Lee ST, Moon J, Sunwoo JS, Byun JI, Lim JA, et al. Tocilizumab in autoimmune encephalitis refractory to rituximab: an institutional cohort study. Neurotherapeutics. 2016;13(4):824–32.
Benucci M, Tramacere L, Infantino M, Manfredi M, Grossi V, Damiani A, et al. Efficacy of tocilizumab in limbic encephalitis with anti-CASPR2 antibodies. Case Rep Neurol Med. 2020;2020:5697670.
Krogias C, Hoepner R, Müller A, Schneider-Gold C, Schröder A, Gold R. Successful treatment of anti-caspr2 syndrome by interleukin 6 receptor blockade through tocilizumab. JAMA Neurol. 2013;70(8):1056–9.
Lee WJ, Lee ST, Shin YW, Lee HS, Shin HR, Kim DY, et al. Teratoma removal, steroid, IVIG, rituximab and tocilizumab (T-SIRT) in Anti-NMDAR encephalitis. Neurotherapeutics. 2021;18(1):474–87.
Randell RL, Adams AV, Van Mater H. Tocilizumab in refractory autoimmune encephalitis: a series of pediatric cases. Pediatric Neurol. 2018;86:66–8.
Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–52.
Vezzani A, Moneta D, Conti M, Richichi C, Ravizza T, De Luigi A, et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci USA. 2000;97(21):11534–9.
Noe FM, Polascheck N, Frigerio F, Bankstahl M, Ravizza T, Marchini S, et al. Pharmacological blockade of IL-1β/IL-1 receptor type 1 axis during epileptogenesis provides neuroprotection in two rat models of temporal lobe epilepsy. Neurobiol Dis. 2013;59:183–93.
Choi CH, Ma SH, Ma KK, Leung H, Mok VC. Super-refractory status epilepticus in autoimmune encephalitis treated with interleukin-1 receptor antagonist, anakinra. Epileptic Disord. 2021;23(3):500–5.
Brunner HI, Quartier P, Alexeeva E, Constantin T, Koné-Paut I, Marzan K, et al. Efficacy and safety of canakinumab in patients with systemic juvenile idiopathic arthritis with and without fever at baseline: results from an open-label. Active-Treatment Extension Study. Arthritis Rheum. 2020;72(12):2147–58.
So A, De Meulemeester M, Pikhlak A, Yücel AE, Richard D, Murphy V, et al. Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: results of a multicenter, phase II, dose-ranging study. Arthritis Rheum. 2010;62(10):3064–76.
Baldwin AG, Brough D, Freeman S. Inhibiting the inflammasome: a chemical perspective. J Med Chem. 2016;59(5):1691–710.
Sjöström EO, Culot M, Leickt L, Åstrand M, Nordling E, Gosselet F, et al. Transport study of interleukin-1 inhibitors using a human in vitro model of the blood-brain barrier. Brain Behav Immun Health. 2021;16: 100307.
DeSena AD, Do T, Schulert GS. Systemic autoinflammation with intractable epilepsy managed with interleukin-1 blockade. J Neuroinflamm. 2018;15(1):38.
Costagliola G, Depietri G, Michev A, Riva A, Foiadelli T, Savasta S, et al. Targeting inflammatory mediators in epilepsy: a systematic review of its molecular basis and clinical applications. Front Neurol. 2022;13: 741244.
van Oosten BW, Barkhof F, Truyen L, Boringa JB, Bertelsmann FW, von Blomberg BM, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology. 1996;47(6):1531–4.
Kondo T, Fukuta M, Takemoto A, Takami Y, Sato M, Takahashi N, et al. Limbic encephalitis associated with relapsing polychondritis responded to infliximab and maintained its condition without recurrence after discontinuation: a case report and review of the literature. Nagoya J Med Sci. 2014;76(3–4):361–8.
Fockaert N, Goffin K, Demaerel P, Van Paesschen W. Infliximab-associated autoimmune limbic encephalitis: a case report. Acta Neurol Belg. 2015;115(2):161–3.
Tofacitinib. Drugs R D. 2010;10(4):271–84.
Jang Y, Lee W-J, Lee H, Chu K, Lee S, Lee S-T. Tofacitinib treatment for refractory autoimmune encephalitis. Epilepsia. 2021;62:e53–9.
Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol. 2015;15(5):283–94.
Lim J-A, Lee S-T, Moon J, Jun J-S, Park B-S, Byun J-I, et al. New feasible treatment for refractory autoimmune encephalitis: low-dose interleukin-2. J Neuroimmunol. 2016;299:107–11.
Close R, Bale P, Gallagher K, Ambegaonkar G, Rossor T, Abbassi N, et al. Poster 137 Growing up on biologics: a case report of development of NMDAr encephalitis in a young person with JIA on abatacept. Rheumatology. 2020;59(Supplement_2):ii68.
Wannamaker W, Davies R, Namchuk M, Pollard J, Ford P, Ku G, et al. (S)-1-((S)-2-{[1-(4-amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an orally available selective interleukin (IL)-converting enzyme/caspase-1 inhibitor, exhibits potent anti-inflammatory activities by inhibiting the release of IL-1beta and IL-18. J Pharmacol Exp Ther. 2007;321(2):509–16.
Maroso M, Balosso S, Ravizza T, Iori V, Wright CI, French J, et al. Interleukin-1β biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics. 2011;8(2):304–15.
Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI). Epilepsy Res. 2013;103(1):2–30.
Yang X-M, Downey JM, Cohen MV, Housley NA, Alvarez DF, Audia JP. The highly selective caspase-1 inhibitor VX-765 provides additive protection against myocardial infarction in rat hearts when combined with a platelet inhibitor. J Cardiovasc Pharmacol Ther. 2017;22(6):574–8.
Li H, Guo Z, Chen J, Du Z, Lu H, Wang Z, et al. Computational research of Belnacasan and new Caspase-1 inhibitor on cerebral ischemia reperfusion injury. Aging (Albany NY). 2022;14(4):1848–64.
Flores J, Noël A, Foveau B, Beauchet O, LeBlanc AC. Pre-symptomatic Caspase-1 inhibitor delays cognitive decline in a mouse model of Alzheimer disease and aging. Nat Commun. 2020;11(1):4571.
Syversen SW, Jørgensen KK, Goll GL, Brun MK, Sandanger Ø, Bjørlykke KH, et al. Effect of therapeutic drug monitoring vs standard therapy during maintenance infliximab therapy on disease control in patients with immune-mediated inflammatory diseases: a randomized clinical trial. JAMA. 2021;326(23):2375–84.
Dhamija R, Eckert S, Wirrell E. Ketogenic diet. Can J Neurol Sci. 2013;40(2):158–67.
Pong AW, Geary BR, Engelstad KM, Natarajan A, Yang H, De Vivo DC. Glucose transporter type I deficiency syndrome: epilepsy phenotypes and outcomes. Epilepsia. 2012;53(9):1503–10.
Andrea-Meira I, Romão TT, Pires do Prado HJ, Krüger LT, Pires MEP, da Conceição PO. Ketogenic Diet and Epilepsy: What We Know So Far. Front Neurosci 2019;13:5.
Ułamek-Kozioł M, Czuczwar SJ, Januszewski S, Pluta R. Ketogenic diet and epilepsy. Nutrients. 2019;11:10.
Rudy L, Carmen R, Daniel R, Artemio R, Moisés RO. Anticonvulsant mechanisms of the ketogenic diet and caloric restriction. Epilepsy Res. 2020;168: 106499.
Murugan M, Boison D. Ketogenic diet, neuroprotection, and antiepileptogenesis. Epilepsy Res. 2020;167: 106444.
Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173(7):1728-41.e13.
Peng A, Qiu X, Lai W, Li W, Zhang L, Zhu X, et al. Altered composition of the gut microbiome in patients with drug-resistant epilepsy. Epilepsy Res. 2018;147:102–7.
Gómez-Eguílaz M, Ramón-Trapero JL, Pérez-Martínez L, Blanco JR. The beneficial effect of probiotics as a supplementary treatment in drug-resistant epilepsy: a pilot study. Benef Microbes. 2018;9(6):875–81.
Carreño M, Bien CG, Asadi-Pooya AA, Sperling M, Marusic P, Elisak M, et al. Epilepsy surgery in drug resistant temporal lobe epilepsy associated with neuronal antibodies. Epilepsy Res. 2017;129:101–5.
Rüegg S. EEG bei Autoimmunenzephalitiden. Zeitschr Epileptol. 2020;33(4):278–87.
Jean WC, Dalmau J, Ho A, Posner JB. Analysis of the IgG subclass distribution and inflammatory infiltrates in patients with anti-Hu-associated paraneoplastic encephalomyelitis. Neurology. 1994;44(1):140–7.
Blaes F, Klotz M, Funke D, Strittmatter M, Kraus J, Kaps M. Disturbance in the serum IgG subclass distribution in patients with anti-Hu positive paraneoplastic neurological syndromes. Eur J Neurol. 2002;9(4):369–72.
Sabater L, Gaig C, Gelpi E, Bataller L, Lewerenz J, Torres-Vega E, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014;13(6):575–86.
Sabater L, Planagumà J, Dalmau J, Graus F. Cellular investigations with human antibodies associated with the anti-IgLON5 syndrome. J Neuroinflamm. 2016;13(1):226.
da Silva-Júnior FP, Castro LH, Andrade JQ, Bastos CG, Moreira CH, Valério RM, et al. Serial and prolonged EEG monitoring in anti-N-Methyl-d-Aspartate receptor encephalitis. Clin Neurophysiol. 2014;125(8):1541–4.
Kaplan PW, Rossetti AO, Kaplan EH, Wieser HG. Proposition: limbic encephalitis may represent limbic status epilepticus. A review of clinical and EEG characteristics. Epilepsy Behav. 2012;24(1):1–6.
Schmitt SE, Pargeon K, Frechette ES, Hirsch LJ, Dalmau J, Friedman D. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology. 2012;79(11):1094–100.
Wang J, Wang K, Wu D, Liang H, Zheng X, Luo B. Extreme delta brush guides to the diagnosis of anti-NMDAR encephalitis. J Neurol Sci. 2015;353(1–2):81–3.
Zhang Y, Liu G, Jiang MD, Li LP, Su YY. Analysis of electroencephalogram characteristics of anti-NMDA receptor encephalitis patients in China. Clin Neurophysiol. 2017;128(7):1227–33.
Ueda J, Kawamoto M, Hikiami R, Ishii J, Yoshimura H, Matsumoto R, et al. Serial EEG findings in anti-NMDA receptor encephalitis: correlation between clinical course and EEG. Epileptic Disord. 2017;19(4):465–70.
Lin N, Huang Y, Jin L, Lu Q, Liu Q, Zhou X, et al. Electroencephalogram and clinical characteristics and correlations in patients with anti-N-Methyl-d-aspartate receptor encephalitis. Clin EEG Neurosci. 2020;51(1):51–60.
Steriade C, Hantus S, Moosa ANV, Rae-Grant AD. Extreme delta—with or without brushes: a potential surrogate marker of disease activity in anti-NMDA-receptor encephalitis. Clin Neurophysiol. 2018;129(10):2197–204.
Chanson E, Bicilli É, Lauxerois M, Kauffmann S, Chabanne R, Ducray F, et al. Anti-NMDA-R encephalitis: should we consider extreme delta brush as electrical status epilepticus? Neurophysiol Clin. 2016;46(1):17–25.
Foff EP, Taplinger D, Suski J, Lopes MB, Quigg M. EEG findings may serve as a potential biomarker for anti-NMDA receptor encephalitis. Clin EEG Neurosci. 2017;48(1):48–53.
Jeannin-Mayer S, André-Obadia N, Rosenberg S, Boutet C, Honnorat J, Antoine JC, et al. EEG analysis in anti-NMDA receptor encephalitis: description of typical patterns. Clin Neurophysiol. 2019;130(2):289–96.
Miao A, Wang X. Ictal rhythmic alpha sinusoidal waves in 3 cases of anti-NMDAR encephalitis. Clin EEG Neurosci. 2018;49(5):302–5.
Limotai C, Denlertchaikul C, Saraya AW, Jirasakuldej S. Predictive values and specificity of electroencephalographic findings in autoimmune encephalitis diagnosis. Epilepsy Behav. 2018;84:29–36.
Gillinder L, Warren N, Hartel G, Dionisio S, O’Gorman C. EEG findings in NMDA encephalitis—a systematic review. Seizure. 2019;65:20–4.
Yao L, Yue W, Xunyi W, Jianhong W, Guoxing Z, Zhen H. Clinical features and long-term outcomes of seizures associated with autoimmune encephalitis: a follow-up study in East China. J Clin Neurosci. 2019;68:73–9.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
Open access funding provided by University of Basel. This review was funded by the Swiss Academy of Medical Sciences (YTCR 11/21; to J.F), the Swiss National Science Foundation (PCEFP3_194609), the National Multiple Sclerosis Society (FG-1708-28871), the Goldschmidt Jacobson Foundation, the Gottfried and Julia Bangerter-Rhyner Foundation, the Propatient Foundation, the Fondation Pierre Mercier pour la Science, and the University of Basel (all to A.-K.P.).
Conflict of Interest
A-K.P. has received speaker honoraria, research support or research/travel support from Roche and Biogen all used for research and without influence on the manuscript. S.R. has received funding from the Swiss National Science Foundation (grant number 320030_169379/1 and co-applicant for grant numbers 33CM30_125115/1 and 33CM30_140338/1, and from UCB-pharma. He has received honoraria from serving on the scientific advisory boards of Arvelle/Angelini, Bial, Eisai, GW, and UCB-pharma, and from serving as a consultant for Arvelle/Angelini, Eisai, Jazz Pharmaceuticals, Pfizer, Novartis, Sandoz, and UCB-pharma. He has received speaker’s honoraria from Eisai and Novartis. He does not hold any stocks of any pharmaceutical industries or manufacturers of medical devices. He disclosed that he is the past-president of the Swiss League against Epilepsy (no payments), Editor-in-chief of Zeitschrift für Epileptologie/ Clinical Epileptology (from 2023) (no payments). J.F., T.N. declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
A-K.P. and S.R. designed the review and supervised the work. J. F., T.N. and S.R. did the initial literature search. A-K.P., J.F. and T.N. drew the figures and composed the tables. All authors drafted and critically revised the manuscript, read and approved the final submitted paper and agree to be accountable for the work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Flammer, J., Neziraj, T., Rüegg, S. et al. Immune Mechanisms in Epileptogenesis: Update on Diagnosis and Treatment of Autoimmune Epilepsy Syndromes. Drugs 83, 135–158 (2023). https://doi.org/10.1007/s40265-022-01826-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01826-9