WO2006094549A1 - A PROCESS FOR THE PREPARATION OF AN 11-(4-SUBSTITUTED-I-PIPERAZINYL)DIBENZO[b,f][1,4]THIAZEPINE DERIVATIVE - Google Patents
A PROCESS FOR THE PREPARATION OF AN 11-(4-SUBSTITUTED-I-PIPERAZINYL)DIBENZO[b,f][1,4]THIAZEPINE DERIVATIVE Download PDFInfo
- Publication number
- WO2006094549A1 WO2006094549A1 PCT/EP2005/014055 EP2005014055W WO2006094549A1 WO 2006094549 A1 WO2006094549 A1 WO 2006094549A1 EP 2005014055 W EP2005014055 W EP 2005014055W WO 2006094549 A1 WO2006094549 A1 WO 2006094549A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- formula
- derivative
- dibenzo
- piperazinyl
- thiazepine
- Prior art date
Links
- JLOAJISUHPIQOX-UHFFFAOYSA-N C(C1)NCCN1C1=Nc2ccccc2Sc2c1cccc2 Chemical compound C(C1)NCCN1C1=Nc2ccccc2Sc2c1cccc2 JLOAJISUHPIQOX-UHFFFAOYSA-N 0.000 description 2
- WFCSWCVEJLETKA-UHFFFAOYSA-N OCCN1CCNCC1 Chemical compound OCCN1CCNCC1 WFCSWCVEJLETKA-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D281/00—Heterocyclic compounds containing rings of more than six members having one nitrogen atom and one sulfur atom as the only ring hetero atoms
- C07D281/02—Seven-membered rings
- C07D281/04—Seven-membered rings having the hetero atoms in positions 1 and 4
- C07D281/08—Seven-membered rings having the hetero atoms in positions 1 and 4 condensed with carbocyclic rings or ring systems
- C07D281/12—Seven-membered rings having the hetero atoms in positions 1 and 4 condensed with carbocyclic rings or ring systems condensed with two six-membered rings
- C07D281/16—[b, f]-condensed
Definitions
- the invention relates to a process for the preparation of an 11-(4- substituted-1-piperazinyl)dibenzo[ ⁇ ,/][1 ,4]thiazepine derivative, of general formula I
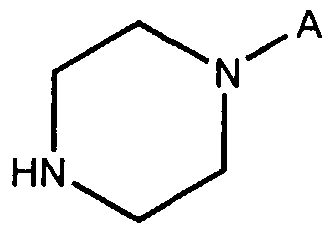
- A is hydrogen or a -(CH 2 J 2 -OH group or a -(CH 2 ) 2 -O-(CH 2 ) 2 -OH group, or of a salt thereof.
- the Formula I compound is quetiapine, a compound known for its dopamine antagonist activity and which may be used as an antipsychotic agent, as a neuroleptic, or in the treatment of hyperactivity, having a notable reduction in some undesirable side effects associated with other active ingredients of the same therapeutical category.
- the Formula I compound when A is hydrogen, the Formula I compound is useful as an intermediate for the preparation of quetiapine itself, as disclosed in EP 0 282 236 B1 , and when A is -(CH 2 ) 2 - OH, the Formula I compound may be easily converted into advanced intermediates for the synthesis of quetiapine, according to the process disclosed in WO 01/055215 A1. State of the Art
- the Formula Il compound is known and the preparation thereof is described, for example, by J. Schmutze et al. HeIv. Chim. Acta, 48, 336 (1965).
- EP 0 282 236 B1 describes a another process for the synthesis of quetiapine, starting out from the intermediate 11-(1- piperazinyl)dibenzo[£>,/][1 ,4]thiazepine, of Formula VIII (see Scheme 3)
- WO 2004/076431 A1 describes a variant synthesis of Scheme 3 in which a phase transfer catalyst is used.
- WO 01/055215 describes another process for the preparation of quetiapine where an 11-(4-(2-haloethyl)-1-piperazinyl)dibenzo[6,/][1 ,4]thiazepine, prepared by ring formation from bicyclic structures, is condensed with ethylene glycol in the presence of metallic sodium.
- A is hydrogen or a -(CH 2 ) 2 -OH group or a -(CH 2 ) 2 -O-(CH 2 ) 2 -OH group, or of a salt thereof, comprising a step in which 10A7-dibenzo- [b,f ⁇ [ ⁇ ,4]thiazepin-11-one of Formula II,
- A has the same meaning as given above, in the presence of a titanium alkoxide, of the general Formula Ti(OR) 4 , where R is a straight or branched C 1 -C 8 alkyl, to obtain said Formula I derivative or a salt thereof.
- the direct reaction of an amide with an amine to give an amidine is unexpected and surprising.
- the present inventors have found very few examples related in the scientific literature and in none of them is a titanium alkoxide used as condensation agent for this type of reactions.
- the reaction proceeds at a high temperature, above 100° C, and the titanium alkoxide acts simultaneously as reactive agent and solvent.
- the Formula Il compound and the piperazine derivative are combined in the final synthesis step. This is particularly advantageous since both products have a high added value, and in this way their use is optimized by reducing the losses for the yields in the different steps.
- the new process for the preparation of a Formula I derivative consists of the direct condensation between 10/-/-dibenzo[ ⁇ ,/][1 ,4]thiazepin-11-one, of Formula II, and a piperazine derivative, of Formula III in the presence of a titanium alkoxide of formula Ti(OR) 4 , where R is a straight or branched alkyl group, having from one to eight carbon atoms (see Scheme 5).
- the titanium alkoxide, of formula Ti(OR) 4 is added at a rate of 1 to 8 equivalents for each equivalent of the Formula Il compound, preferably at a rate of 1.5 to 4 equivalents for each equivalent of the Formula Il compound, and most preferably at a rate of 2.8 to 3.2 equivalents for each equivalent of the Formula Il compound.
- R is ethyl, isopropyl or n-butyl.
- the piperazine derivative, of Formula III is added at a rate of 1.5 to 4 equivalents for each equivalent of the Formula Il compound, preferably at a rate of 1.8 to 2.2 equivalents for each equivalent of the Formula Il compound.
- the molar ratio between the titanium alkoxide, of formula Ti(OR) 4 , and the piperazine derivative, of Formula III ranges from 1 :1.5 to 5:1 , preferably from 1 :1.2 to 2.7:1 and most preferably from 1 :1.1 and 1.8:1.
- the reaction step of the Formula Il compound with the piperazine derivative is conducted at a temperature ranging from 140 0 C to 200 0 C, preferably from 160°C to 190°C and most preferably from 165°C and 175 0 C.
- the distillation, at least partial, of the alcohol generated during the reaction is carried out simultaneously during that step.
- At least one of the following compounds may be used as an additive: an alcohol having a boiling point above the reaction temperature, a molecular sieve, silica, acetic acid, pyridine, 2,6-dimethylpiperidine or 2- methylpiperidine.
- an alcohol having a boiling point above the reaction temperature e.g., a molecular sieve, silica, acetic acid, pyridine, 2,6-dimethylpiperidine or 2- methylpiperidine.
- a solid having a melting point of 200-210 0 C it may be advantageous to use a high boiling point alcohol as diluent.
- a particularly preferred embodiment of the process of the invention is obtained when, with R being isopropyl, the titanium tetraisopropoxide, of formula Ti(O/Pr) 4 , is added at a rate of 1.5 to 4 equivalents for each equivalent of the Formula Il compound, the piperazine derivative, of Formula III, is added at a rate of 1.5 to 4 equivalents for each equivalent of the Formula Il compound, the molar ratio between Ti(O/Pr) 4 , and the piperazine derivative ranges from 1 :1.2 to 2.7:1 , the reaction step is conducted at a temperature ranging from 16O 0 C to 190 0 C, with a reaction time ranging from 3 to 12 hours, and during said reaction step the isopropanol generated during the reaction is simultaneously distilled off, at least in part.
- Another particularly preferred embodiment of the process of the invention is obtained when, with R being isopropyl, the titanium tetraisopropoxide, of formula Ti(OZPr) 4 , is added at a rate of 2.8 to 3.2 equivalents for each equivalent of the Formula Il compound, the piperazine derivative, of Formula III, is added at a rate of 1.8 to 2.2 equivalents for each equivalent of the Formula Il compound, the molar ratio between Ti(CvPr) 4 , and the piperazine derivative ranges from 1 :1.1 to 1.8:1 , the reaction step is conducted at a temperature ranging from 165 0 C to 175°C, with a reaction time ranging from 4 to 6 hours, and during said reaction step the isopropanol generated during the reaction is simultaneously distilled off, at least in part.
- the piperazine derivative is 1-(2-(2- hydroxyethoxy)ethyl)piperazine, of Formula V,
- 11-(4-substituted-1-piperazinyl)dibenzo[b,/][1 ,4]thiazepine derivative is 11-(4-(2-(2-hydroxyethoxy)ethyl)-1- piperazinyOdibenzofo/jn ⁇ thiazepine (quetiapine) of Formula Vl.
- the process comprises an additional step of reacting the quetiapine with fumaric acid to produce quetiapine hemifumarate.
- the piperazine derivative is piperazine
- the 11-(4- substituted-1-piperazinyl)dibenzo[b,/][1,4]thiazepine is 11-(1- piperazinyl)dibenzo[b,r][1 ,4]thiazepine, of Formula VIII.
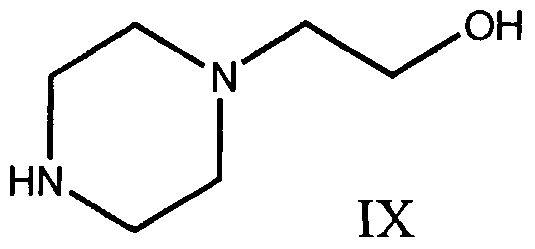
- the piperazine derivative is 1-(2-hydroxyethyl)piperazine, of Formula IX,
- the titanium reactive agent was destroyed with an excess of water and the precipitate was removed from the mixture by filtration. After washing the residues with toluene, the combined filtrates were washed with water and concentrated under vacuum to give a mixture of quetiapine (Vl) and 1-heptanol (4.24 g) as a yellow oil.
- the quetiapine was isolated as the hemifumarate salt thereof (2.295 g, 75%) after crystallization with the process described herebelow.
Abstract
A process for the preparation of an 11-(4-substituted-1-piperazinyl)dibenzo[b,f][1,4]thiazepine derivative, of general Formula (I), where A is hydrogen or a -(CH2)2-OH group or a -(CH2)2-0-(CH2)2-OH group, or of a salt thereof, comprises a step in which 10H-dibenzo[b,f][1,4]thiazepin-11-one is reacted with a piperazine derivative in the presence of a titanium alkoxide of general formula Ti(OR)4, where R is a straight or branched alkyl group, having from one to eight carbon atoms to obtain said Formula I derivative or a salt thereof. Where A is -(CH2)2-0-(CH2)2-OH, then the piperazine derivative is 1-(2-(2-hydroxyethoxy)ethyl)piperazine and the 11-(4-substituted-1-piperazinyl)dibenzo[b,f][1,4]thiazepine is quetiapine, (11-(4-(2-(2-hydroxyethoxy)ethyl)-1-piperazinyl)dibenzo[b,f][1,4]thiazepine). The process may comprise an additional step of reacting the quetiapine with fumaric acid to obtain quetiapine hemifumarate.
Description
A PROCESS FOR THE PREPARATION OF AN
1 1 -(4-SUBSTITUTED-I -PIPERAZINYL)DIBENZO[M[I ^]THIAZEPINE
DERIVATIVE
DESCRIPTION
Field of the Invention
The invention relates to a process for the preparation of an 11-(4- substituted-1-piperazinyl)dibenzo[Λ,/][1 ,4]thiazepine derivative, of general formula I
where A is hydrogen or a -(CH2J2-OH group or a -(CH2)2-O-(CH2)2-OH group, or of a salt thereof.
When A is the -(CH2)2-O-(CH2)2-OH group, the Formula I compound is quetiapine, a compound known for its dopamine antagonist activity and which may be used as an antipsychotic agent, as a neuroleptic, or in the treatment of hyperactivity, having a notable reduction in some undesirable side effects associated with other active ingredients of the same therapeutical category. On the other hand, when A is hydrogen, the Formula I compound is useful as an intermediate for the preparation of quetiapine itself, as disclosed in EP 0 282 236 B1 , and when A is -(CH2)2-
OH, the Formula I compound may be easily converted into advanced intermediates for the synthesis of quetiapine, according to the process disclosed in WO 01/055215 A1. State of the Art
Several processes are known for the synthesis of 11-(4-substituted-1- piperazinyl)dibenzo[ύ,/][1,4]thiazepine derivatives, of general formula I, and in particular of quetiapine. For example, in EP 0 240 228 B1 two alternative processes for the synthesis of quetiapine (see schemes 1 and 2) are described.
Scheme 1
POCU
In general these processes start out from 10/-/-dibenzo-[ό,/][1 l4]thiazepin- 11-one, of Formula II, and require the synthesis of an halogenated intermediate, of Formula IV, or a sulphur derivative, of Formulas VII or Xl.
C1-3 alkyl
The Formula Il compound is known and the preparation thereof is described, for example, by J. Schmutze et al. HeIv. Chim. Acta, 48, 336 (1965).
EP 0 282 236 B1 describes a another process for the synthesis of quetiapine, starting out from the intermediate 11-(1- piperazinyl)dibenzo[£>,/][1 ,4]thiazepine, of Formula VIII (see Scheme 3)
Scheme 3
Nevertheless, the preparation of said Formula VIII compound again requires the synthesis of a halogenated intermediate (see Scheme 4)
Scheme 4
WO 2004/076431 A1 describes a variant synthesis of Scheme 3 in which a phase transfer catalyst is used.
WO 01/055215 describes another process for the preparation of quetiapine where an 11-(4-(2-haloethyl)-1-piperazinyl)dibenzo[6,/][1 ,4]thiazepine, prepared by ring formation from bicyclic structures, is condensed with ethylene glycol in the presence of metallic sodium.
Summary of the Invention
It is an object of the invention to provide a new synthetic process for the preparation of an 11-(4-sϋbstituted-1-piperazinyl)dibenzo[£>,/][1 ,4]thiazepine derivative of general Formula I1
where A is hydrogen or a -(CH2)2-OH group or a -(CH2)2-O-(CH2)2-OH group, or of a salt thereof, comprising a step in which 10A7-dibenzo- [b,f\[\ ,4]thiazepin-11-one of Formula II,
III
where A has the same meaning as given above, in the presence of a titanium alkoxide, of the general Formula Ti(OR)4, where R is a straight or branched C1-C8 alkyl, to obtain said Formula I derivative or a salt thereof.
In the process of the invention, the direct reaction of an amide with an amine to give an amidine is unexpected and surprising. The present inventors have found very few examples related in the scientific literature and in none of them is a titanium alkoxide used as condensation agent for this type of reactions. The reaction proceeds at a high temperature, above 100° C, and the titanium alkoxide acts simultaneously as reactive agent and solvent.
The process according to the invention effectively affords a number of advantages.
a) Reduction of synthesis steps.
The direct reaction between the Formula Il compound and the piperazine derivative of general Formula III allows the number of synthesis steps to be reduced in comparison with the known prior art processes. For example, some of the known prior art processes stated above are, in summary:
EP 0 240 228 B1 i Il + POCI3 = IV ii IV + V = Vl (quetiapine)
or i Il + P2S5 = VII ii VII + CH3I = Xl (R=CH3) iii Xl + V = Vl (quetiapine)
EP 0 282 236 B1 i. Il + POCI3 = IV ii. IV + piperazine = VIII
Ni. VIII + CICH2CH2OCH2CH2OH = Vl (quetiapine)
b) Convergence
In the process of the invention, the Formula Il compound and the piperazine derivative are combined in the final synthesis step. This is particularly advantageous since both products have a high added value, and in this way their use is optimized by reducing the losses for the yields in the different steps.
c) Lower consumption of the piperazine derivative equivalents
In the process of the invention, there is avoided the need to use, at least, two equivalents of the piperazine derivative, with a view to neutralizing the hydrogen chloride formed as by-product in the processes using the chlorinated derivative of Formula IV. This allows the consumption of said piperazine derivative to be reduced.
d) Working conditions and environemental effects
In the prior art processes it is always necessary to use highly toxic and hazardous reactants, such as for example phosphorous oxichloride, phosphorous pentachloride or phosphorous pentasulfide. Also to be highlighted is the generation of toxic by-products such as, for example, hydrogen chloride, methylmercaptan, phosphoric acid or hydrochloric acid. Nevertheless, in the process of the invention, both the reactive agent used,
a titanium alkoxide, and the by-products formed, titanium dioxide and a lower alcohol, are much easier to handle, have low toxicity, are not chlorinated and are much less harmful for the environment.
Detailed Description of Embodiments of the Invention
The new process for the preparation of a Formula I derivative consists of the direct condensation between 10/-/-dibenzo[ύ,/][1 ,4]thiazepin-11-one, of Formula II, and a piperazine derivative, of Formula III in the presence of a titanium alkoxide of formula Ti(OR)4, where R is a straight or branched alkyl group, having from one to eight carbon atoms (see Scheme 5).
Advantageously, the titanium alkoxide, of formula Ti(OR)4, is added at a rate of 1 to 8 equivalents for each equivalent of the Formula Il compound, preferably at a rate of 1.5 to 4 equivalents for each equivalent of the Formula Il compound, and most preferably at a rate of 2.8 to 3.2 equivalents for each equivalent of the Formula Il compound. Preferably R is ethyl, isopropyl or n-butyl.
Advantageously, the piperazine derivative, of Formula III, is added at a rate of 1.5 to 4 equivalents for each equivalent of the Formula Il compound, preferably at a rate of 1.8 to 2.2 equivalents for each equivalent of the Formula Il compound.
Advantageously, the molar ratio between the titanium alkoxide, of formula Ti(OR)4, and the piperazine derivative, of Formula III, ranges from 1 :1.5 to 5:1 , preferably from 1 :1.2 to 2.7:1 and most preferably from 1 :1.1 and 1.8:1.
Advantageously, the reaction step of the Formula Il compound with the piperazine derivative is conducted at a temperature ranging from 1400C to 2000C, preferably from 160°C to 190°C and most preferably from 165°C and 1750C. Advantageously the distillation, at least partial, of the alcohol
generated during the reaction is carried out simultaneously during that step.
Scheme 5
Fumaric acid EtOH
Optionally, at least one of the following compounds may be used as an additive: an alcohol having a boiling point above the reaction temperature, a molecular sieve, silica, acetic acid, pyridine, 2,6-dimethylpiperidine or 2- methylpiperidine. For example, when titanium tetramethoxide is used, a
solid having a melting point of 200-2100C, it may be advantageous to use a high boiling point alcohol as diluent.
A particularly preferred embodiment of the process of the invention is obtained when, with R being isopropyl, the titanium tetraisopropoxide, of formula Ti(O/Pr)4, is added at a rate of 1.5 to 4 equivalents for each equivalent of the Formula Il compound, the piperazine derivative, of Formula III, is added at a rate of 1.5 to 4 equivalents for each equivalent of the Formula Il compound, the molar ratio between Ti(O/Pr)4, and the piperazine derivative ranges from 1 :1.2 to 2.7:1 , the reaction step is conducted at a temperature ranging from 16O0C to 1900C, with a reaction time ranging from 3 to 12 hours, and during said reaction step the isopropanol generated during the reaction is simultaneously distilled off, at least in part.
Another particularly preferred embodiment of the process of the invention is obtained when, with R being isopropyl, the titanium tetraisopropoxide, of formula Ti(OZPr)4, is added at a rate of 2.8 to 3.2 equivalents for each equivalent of the Formula Il compound, the piperazine derivative, of Formula III, is added at a rate of 1.8 to 2.2 equivalents for each equivalent of the Formula Il compound, the molar ratio between Ti(CvPr)4, and the piperazine derivative ranges from 1 :1.1 to 1.8:1 , the reaction step is conducted at a temperature ranging from 1650C to 175°C, with a reaction time ranging from 4 to 6 hours, and during said reaction step the isopropanol generated during the reaction is simultaneously distilled off, at least in part.
Preferably, the piperazine derivative is 1-(2-(2- hydroxyethoxy)ethyl)piperazine, of Formula V,
and the 11-(4-substituted-1-piperazinyl)dibenzo[b,/][1 ,4]thiazepine derivative is 11-(4-(2-(2-hydroxyethoxy)ethyl)-1- piperazinyOdibenzofo/jn ^thiazepine (quetiapine) of Formula Vl.
VI
This corresponds to the particular case in which, in the Formula I compound, A is -(CH2)2-O-(CH2)2-OH. Advantageously, the process comprises an additional step of reacting the quetiapine with fumaric acid to produce quetiapine hemifumarate.
Preferably, the piperazine derivative is piperazine, and the 11-(4- substituted-1-piperazinyl)dibenzo[b,/][1,4]thiazepine is 11-(1- piperazinyl)dibenzo[b,r][1 ,4]thiazepine, of Formula VIII.
VIII
This corresponds to the particular case in which, in the Formula compound, A is hydrogen.
Preferably the piperazine derivative is 1-(2-hydroxyethyl)piperazine, of Formula IX,
and the 1 1-(4-substituted-1-piperazinyl)dibenzo[b,/][1 ,4]thiazepine is 1 1-(4- (2-hydroxyethyl)-1-piperazinyl)dibenzo[ύ,f][1 ,4]thiazepine, of Formula X,
This corresponds to the particular case in which, in the Formula I compound, A is — (CH2)2-OH.
EXAMPLES
Example 1
To a mixture of 10H-dibenzo-[ό,/][1 ,4]thiazepin-11-one (II) (68.1 g, 300 mmol) and 1-(2-(2-hydroxyethoxy)ethyl)piperazine (V) (157 g, 900 mmol) there was added titanium tetraisopropoxide (247 ml, 830 mmol) under an inert atmosphere. The resulting suspension was heated to 1700C and the isopropanol generated during the reaction was removed by distillation.
When the distillation seemed to terminate, a gentle vacuum was applied for 5 minutes to complete it. After 6 hours reaction, the mixture was cooled to 1000C and diluted with toluene. The titanium reactive agent was destroyed with an excess of water and the precipitate was filtered off. After washing the residue twice with toluene, the combined filtrates were washed twice with water and concentrated under vacuum to give quetiapine (V) (98.5 g, 86%) as a viscous yellow oil.
IR 3398, 2919, 2855, 1597, 1575, 1558, 1456, 1437, 1306, 1250, 1244, 11 18, 1063, 1014, 762 and 742 cm"1.
1H NMR (400 MHz, CDCI3) 7.52-7.49 (m, 1 H), 7.40-7.37 (m, 1 H), 7.35- 7.28 (m, 3 H), 7.19-7.15 (m, 1 H), 7.08-7.05 (m, 1 H), 6.90-6.86 (m, 1 H), 3.71-3.61 (m, 10 H), 3.50 (brs, 1 H), 2.65-2.62 (m, 4 H) and 2.58-2.54 (m, 2 H).
13C NMR (101 MHz, CDCI3) 160.6, 148.9, 140.0, 134.1 , 132.2, 132.1 , 130.8, 129.1 , 128.9, 128.3, 127.9, 125.3, 122.8, 72.4, 67.7, 62.0, 58.0, 53.1 and 46.5.
m/z (El) 383 (M+, 4%), 321 (M - HOCH2CH2OH, 50), 239 (M - HOCH2CH2OH - HOCH2, 65) and 210 (dibenzothiazepine - H, 100).
Example 2
To a mixture of 10H-dibenzo-[b,r][1 ,4]thiazepin-11-one (II) (454 mg, 2 mmol) and 1-(2-(2-hydroxyethoxy)ethyl)piperazine (V) (522 mg, 3 mmol) there was added titanium tetraisopropoxide (2.4 ml, 8 mmol) under an inert atmosphere. The resulting suspension was heated to 2000C and the isopropanol generated during the reaction was removed by distillation. After 20 hours reaction, the mixture was cooled to 1000C and diluted with toluene. The titanium reactive agent was destroyed with an excess of water and the precipitate was removed from the mixture by filtration. After
washing the residues twice with toluene, the combined filtrates were washed twice with water and concentrated under vacuum to give quetiapine (Vl) (465 mg, 61%) as a viscous yellow oil having identical characteristics as those described for Example 1.
Example 3
To a mixture of 10/-/-dibenzo-[6,/][1 ,4]thiazepin-11-one (II) (22.7 g, 100 mmol) and 1-(2-(2-hydroxyethoxy)ethyl)piperazine (V) (31.3 g, 180 mmol) there was added titanium tetraisopropoxide (50.5 ml, 170 mmol) under an inert atmosphere. The resulting suspension was heated to 170°C and the isopropanol generated during the reaction was removed by distillation. When the distillation was ending, a gentle vacuum was applied for 5 minutes to complete the distillation. After 6 hours total reaction time, the mixture was cooled to 1000C and diluted with toluene. The titanium reactive agent was destroyed with an excess of water and the precipitate was removed from the mixture by filtration. After washing the residues twice with toluene, the combined filtrates were washed twice with water and concentrated under vacuum to give quetiapine (Vl) (35.9 g, 94%) as a viscous yellow oil having identical characteristics as those described for Example 1.
Example 4
To a mixture of 10H-dibenzo-[b,/][1,4]thiazepin-11-one (II) (68.1 g, 300 mmol) and 1-(2-(2-hydroxyethoxy)ethyl)piperazine (V) (104.4 g, 600 mmol) there was added titanium tetraisopropoxide (268 ml, 900 mmol) under an inert atmosphere. The resulting suspension was heated to 1700C and the isopropanol generated during the reaction was removed by distillation. When the distillation was ending, a gentle vacuum was applied for 5 minutes to complete the distillation. After 6 hours total reaction time, the mixture was cooled to 100°C and diluted with toluene. The titanium reactive agent was destroyed with an excess of water and the precipitate was
removed from the mixture by filtration. After washing the residues twice with toluene, the combined filtrates were washed twice with water and concentrated under vacuum to give quetiapine (V) (100.0 g, 87%) as a viscous yellow oil having identical characteristics as those described for Example 1.
Example 5
To a mixture of 10/-/-dibenzo-[/b,/][1 ,4]thiazepin-11-one (||) (2.27 g, 10 mmol), 1-(2-(2-hydroxyethoxy)ethyl)piperazine (V) (2.61 g, 15 mmol) and 1- heptanol (4.2 ml, 30 mmol) there was added titanium tetraisopropoxide (8.0 ml, 27 mmol) under an inert atmosphere. The resulting suspension was heated to 160°C and the isopropanol generated during the reaction was removed by distillation. After 32 hours the mixture was cooled to 1000C and diluted with toluene. The titanium reactive agent was destroyed with an excess of water and the precipitate was removed from the mixture by filtration. After washing the residues with toluene, the combined filtrates were washed with water and concentrated under vacuum to give a mixture of quetiapine (Vl) and 1-heptanol (4.24 g) as a yellow oil. The quetiapine was isolated as the hemifumarate salt thereof (2.295 g, 75%) after crystallization with the process described herebelow.
Example 6
To a solution of quetiapine (Vl) (82.5 g, 215 mmol) in ethanol (660 ml) there was added fumaric acid (12.49 g, 108 mmol) and the mixture was heated under reflux for 2 hours in an inert atmosphere. A precipitate out of the solution formed shortly after starting the heating. After cooling the mixture to O0C, it was filtered, washed with ethanol (250 ml, 00C) and dried at reduced pressure to give quetiapine hemifumarate (77.4 g, 81%) as a crystalline white solid.
Melting point: 173-175°C
IR 3315, 2944, 2869, 1600, 1572, 1459, 1413, 1335, 1306, 1130, 1082, 1064, 989, 794 and 768 cm"1.
1H NMR (400 MHz, CDCI3/CD3OD) 7.52 (d, J = 7.6 Hz, 1 H), 7.40 (dd, J = 8.0 and 1.6 Hz, 1 H), 7.38-7.31 (m, 2 H), 7.19 (m, 1 H), 7.08 (dd, J = 8.0 and 1.2 Hz, 1 H), 6.92 (m, 1 H), 6.78 (s, 1 H), 3.87 (s, 4 H), 3.73 (m, 2 H), 3.67 (m, 2 H), 3.55 (m, 2 H), 3.03 (brm, 2 H) and 2.94-2.89 (m, 4 H).
13C NMR (101 MHz, CDCI3/CD3OD) 170.1 , 160.3, 148.3, 139.8, 135.1 , 133.4, 132.11 , 132.09, 131.1 , 129.1 , 128.8, 128.5, 127.9, 125.1 , 123.3, 72.5, 65.9, 61.0, 57.2, 52.1 and 45.1.
m/z (Cl, NH3) 384 (MH+, 100%).
Example 7
To a mixture of 10/-/-dibenzo-[b,/][1 ,4]thiazepin-11-one (II) (2 g, 8.8 mmol) and piperazine (2.27 g, 26.4 mmol) there was added titanium tetraisopropoxide (7.0 ml, 25.8 mmol) under an inert atmosphere. The resulting suspension was heated to 1700C and the isopropanol generated during the reaction was removed by distillation. After 5 hours the mixture was cooled to 1000C and diluted with toluene. The titanium reactive agent was destroyed with an excess of water and the precipitate was removed from the mixture by filtration. After washing the residues with toluene, the combined filtrates were washed with water and concentrated under vacuum to give 11-(1-piperazinyl)dibenzo-fjb,/][1 ,4-thiazepine (VIII) (2.58 g, 99%) as a viscous yellow oil. After purification by silica gel column chromatography (eluent: dichloromethane to dichloromethane/methanol 95:5), 1.55 g (60%) of 11-(1-piperazinyl)dibenzo-[/3,/)[1I4]-thiazepine (VIII) of high purity were obtained as a colourless viscous oil.
Rf (SiO2, CH2CI2/Me0H 9:1) 0.13
IR 3316, 3051 , 2940, 2846, 1596, 1573, 1555, 1474, 1453, 1409, 1318, 1301 , 1243, 1141 , 1018, 762 and 741 cm'1.
1H NMR (400 MHz, CDCI3) 7.52-7.49 (m, 1 H), 7.39 (dd, J = 7.6 and 1.6 Hz, 1 H), 7.34-7.28 (m, 3 H), 7.19-7.14 (m, 1 H), 7.07 (dd, J = 8.0 and 1.2 Hz, 1 H), 6.88 (ddd, J = 7.2, 7.2 and 1.6 Hz, 1 H), 3.49 (brs, 4 H), 2.97 (brs, 2 H), 2.92-2.86 (m, 2 H) and 1.91 (brs, 1 H).
13C NMR (101 MHz, CDCI3) 161.0, 148.9, 139.9, 134.1, 132.11 , 132.09, 130.7, 129.0, 128.9, 128.2, 127.9, 125.3, 122.7, 48.0 and 45.9.
m/z (Cl, NH3) 296 (MH+, 100%) and 227 (dibenzothiazepine - H/NH3, 16).
Example 8
To a mixture of 10H-dibenzo-[b,/][1 ,4]thiazepin-11-one (II) (2.2 g, 9.7 mmol) and 1-(2-hydroxyethyl)piperazine (IX) (2.5 g, 19.2 mmol) there was added titanium tetraisopropoxide (9.0 ml, 30.3 mmol) under an inert atmosphere. The resulting suspension was heated to 17O0C and the isopropanol generated during the reaction was removed by distillation. After 5.5 hours the mixture was cooled to 1000C and diluted with toluene. The titanium reactive agent was destroyed with an excess of water and the precipitate was removed from the mixture by filtration. After washing the residues with toluene, the combined filtrates were washed with water and concentrated under vacuum to give 11-(4-(2-hydroxyethyl)-1- piperazinyl)dibenzo-[/?,/][1 ,4-thiazepine (X) (3.0 g, 91%) as a yellow oil. After purification by silica gel column chromatography (eluent: dichloromethane to dichloromethane/methanol 97:3), 1.64 g (50%) of 11- (4-(2-hydroxyethyl)-1-piperazinyl)dibenzo-[b,f][1 ,4-thiazepine (X) of high purity were obtained as a colourless viscous oil.
Rf (SiO2, CH2CI2/Me0H 9:1) 0.43
I R 3406, 2936, 2814, 1598, 1574, 1453, 1408, 1305, 1256, 1146, 1061 , 1011 , 762 and 741 cm".
H NMR (400 MHz, CDCI3) 7.52-7.50 (m, 1 H), 7.39 (dd, J = 7.6 and 1.2 Hz, 1 H), 7.35-7.27 (m, 3 H), 7.19-7.15 (m, 1 H), 7.08 (dd, J = 8.0 and 1.6 Hz, 1 H), 6.88 (ddd, J = 7.6, 7.6 and 1.6 Hz, 1 H), 3.65 (t, J = 5.6 Hz, 2 H), 3.56 (brs, 4 H), 2.64 (brs, 1 H) and 2.61-2.52 (m, 6 H).
13C NMR (101 MHz1 CDCI3) 160.7, 148.8, 139.9, 134.1 , 132.1 , 130.8, 129.1 , 128.9, 128.2, 127.9, 125.3, 122.9, 59.4, 57.7, 52.7, 46.8 and 29.6.
/77/Z (CI, NH3) 340 (MN+, 100%) and 228 (dibenzothiazepine / NH3, 21 ).
Claims
1.- A process for the preparation of an 11-(4-substituted-1- piperazinyl)dibenzo[b,/][1 ,4]thiazepine derivative, of general formula I
where A is hydrogen or a -(CH2)2-OH group or a -(CH2)2-O-(CH2)2-OH group, or of a salt thereof, comprising a step in which 10/-/-dibenzo- [b,/][1 ,4]-thiazepin-11-one of Formula II,
is directly reacted with a piperazine derivative, of general Formula
III where A has the same meaning as given above, in the presence of a titanium alkoxide, of the general Formula Ti(OR)4, where R is a straight or branched alkyl, having one to eight carbon atoms, to obtain said Formula I derivative or a salt thereof.
2.- The process of claim 1 wherein R is ethyl, isopropyl, or n-butyl.
3.- The process of claim 1 wherein R is isopropyl.
4.- The process of any one of claims 1 to 3, wherein said titanium alkoxide, of formula Ti(OR)4, is added at a rate of 1 to 8 equivalents for each equivalent of the Formula Il compound, preferably at a rate of 1.5 to 4 equivalents for each equivalent of the Formula Il compound, and most preferably at a rate of 2.8 to 3.2 equivalents for each equivalent of the Formula Il compound.
5.- The process of any one of claims 1 to 4, wherein said piperazine derivative, of Formula III, is added at a rate of 1.5 to 4 equivalents for each equivalent of the Formula Il compound, preferably at a rate of 1.8 to 2.2 equivalents for each equivalent of the Formula Il compound.
6.- The process of any one of claims 1 to 5, wherein the molar ratio between said titanium alkoxide, of formula Ti(OR)4, and said piperazine derivative, of Formula III, ranges from 1 :1.5 to 5:1 , preferably from 1 :1.2 to 2.7:1 and most preferably from 1 :1.1 to 1.5:1.
7.- The process of any one of claims 1 to 6, wherein said step is conducted at a temperature ranging from 14O0C to 2000C, preferably from 160°C to 19O0C and most preferably from 1650C and 175°C.
8.- The process of any one of claims 1 to 7, wherein the distillation, at least partial, of the alcohol generated as by-product during the reaction is carried out simultaneously during said step.
9.- The process of any one of claims 1 to 8, wherein there is added as additive an alcohol having a boiling point above the reaction temperature, a molecular sieve, silica, acetic acid, pyridine, 2,6-dimethylpiperidine or 2- methylpiperidine.
10.- The process of claim 1 , wherein when R is isopropyl, said titanium tetraisopropoxide, of formula Ti(OZPr)4, is added at a rate of 1.5 to 4 equivalents for each equivalent of the Formula Il compound, said piperazine derivative, of Formula III, is added at a rate of 1.5 to 4 equivalents for each equivalent of the Formula Il compound, said molar ratio between Ti(OZPr)4 and said piperazine derivative ranges from 1 :1.2 to 2.7:1 , said step is conducted at a temperature ranging from 1600C to 1900C, said step has a reaction time ranging from 3 to 12 hours, and during said step there is carried out simultaneously the at least partial distillation of the isopropanol generated during the reaction.
11.- The process of claim 1 , wherein when R is isopropyl, said titanium tetraisopropoxide, of formula Ti(OZPr)4, is added at a rate of 2.8 to 3.2 equivalents for each equivalent of the Formula Il compound, said piperazine derivative, of Formula III, is added at a rate of 1.8 to 2.2 equivalents for each equivalent of the Formula Il compound, said molar ratio between Ti(OZPr)4 and said piperazine derivative ranges from 1 :1.1 to 1.8:1 , said step is conducted at a temperature ranging from 165°C to 175°C, said step has a reaction time ranging from 4 to 6 hours, and during said step there is carried out simultaneously the at least partial distillation of the isopropanol generated during the reaction.
12.- The process of any one of claims 1 to 11 , wherein said piperazine derivative is 1-(2-(2-hydroxyethoxy)ethyl)piperazine, of Formula V
and said 11-(4-substituted-1-piperazinyl)dibenzorjb,/][1 ,4]thiazepine derivative is 11-(4-(2-(2-hydroxyethoxy)ethyl)-1- piperazinyl)dibenzo[/),/][1 ,4]thiazepine, of Formula Vl
VI
13.- The process of claim 12, comprising a step of reacting said 11-(4- (2-(2-hydroxyethoxy)ethyl)-1-piperazinyl)dibenzoFjb,/][1 ,4]thiazepine, of Formula Vl, with fumaric acid to obtain quetiapine hemifumarate.
14.- The process of any one of claims 1 to 11 , wherein said piperazine derivative es piperazine, and said 11-(4-substituted-1- piperazinyl)dibenzo[/),fl[1,4]thiazepine derivative is 11-(1- piperazinyl)dibenzo[/),/][1 ,4]thiazepine, of Formula VIII
VIII
15.- The process of any one of claims 1 to 11 , wherein said piperazine derivative is 1-(2-hydroxyethyl)piperazine, of Formula IX,
and said 11-(4-substituted-1-piperazinyl)dibenzo[fc,/][1 ,4]thiazepine derivative is 11-(4-(2-hydroxyethyl)-1- piperazinyl)dibenzo[/j,fl[1 ,4]thiazepine, of Formula X.
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP05850361A EP1856073B1 (en) | 2005-03-07 | 2005-12-21 | A PROCESS FOR THE PREPARATION OF AN 11-(4-SUBSTITUTED-I-PIPERAZINYL)DIBENZO[b,f][1,4]THIAZEPINE DERIVATIVE |
| AT05850361T ATE467624T1 (en) | 2005-03-07 | 2005-12-21 | METHOD FOR PRODUCING A 11-(4-SUBSTITUTED 1-PIPERAZINYL)DIBENZOÄB,FÜÄ1, 4ÜTHIAZEPINE DERIVATIVE |
| DE602005021256T DE602005021256D1 (en) | 2005-03-07 | 2005-12-21 | PROCESS FOR PREPARING AN 11- (4-SUBSTITUTED 1-PIPERAZINYL) DIBENZOEA, FUELEDIETIAZEPINE DERIVATIVE |
| DK05850361.6T DK1856073T3 (en) | 2005-03-07 | 2005-12-21 | Process for the preparation of an 11- (4-substituted-1-piperazinyl) dibenzo [b, f] [1,4] thiazepine derivative |
| US11/817,884 US7902355B2 (en) | 2005-03-07 | 2005-12-21 | Process for the preparation of an 11-(4-substituted-1-piperazinyl)dibenzo[b,f][1,4]thiazepine derivative |
| NO20075071A NO20075071L (en) | 2005-03-07 | 2007-10-08 | Process for Preparation of an 11- (4-Substituted-1-Piperazinyl) Dibenzo [b, f] [1,4] Thiazepine Derivate 2005 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ES200500513 | 2005-03-07 | ||
| ES200500513A ES2234447B1 (en) | 2005-03-07 | 2005-03-07 | PROCEDURE FOR OBTAINING A DERIVATIVE OF 11- (4-SUBSTITUTED-1-PIPERAZINIL) DIBENZO (B, F) (1,4) THIAZEPINE. |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2006094549A1 true WO2006094549A1 (en) | 2006-09-14 |
Family
ID=34707636
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP2005/014055 WO2006094549A1 (en) | 2005-03-07 | 2005-12-21 | A PROCESS FOR THE PREPARATION OF AN 11-(4-SUBSTITUTED-I-PIPERAZINYL)DIBENZO[b,f][1,4]THIAZEPINE DERIVATIVE |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US7902355B2 (en) |
| EP (1) | EP1856073B1 (en) |
| AT (1) | ATE467624T1 (en) |
| DE (1) | DE602005021256D1 (en) |
| DK (1) | DK1856073T3 (en) |
| ES (1) | ES2234447B1 (en) |
| NO (1) | NO20075071L (en) |
| PT (1) | PT1856073E (en) |
| WO (1) | WO2006094549A1 (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CZ300451B6 (en) * | 2006-07-03 | 2009-05-20 | Farmak, A. S. | Process for preparing salts of 2-[2-(4-dibenzo[b,f][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]ethanol (quetiapine) and purification process of such salts |
| US7687622B2 (en) | 2005-04-14 | 2010-03-30 | Teva Pharmaceutical Industries, Ltd | Process for preparing quetiapine fumarate |
| WO2010100623A1 (en) | 2009-03-04 | 2010-09-10 | Ranbaxy Laboratories Limited | Process for the preparation of quetiapine fumarate |
| JP2011510960A (en) * | 2008-01-31 | 2011-04-07 | フェルミオン オサケ ユキチュア | Method for producing quetiapine |
| CN103664822A (en) * | 2012-09-18 | 2014-03-26 | 四川大学华西医院 | Compound used as anesthetic |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101891707B (en) * | 2010-07-29 | 2012-10-17 | 浙江海正药业股份有限公司 | Method for preparing Quetiapine or pharmaceutically acceptable fumarate thereof |
| CN104803947A (en) * | 2015-03-12 | 2015-07-29 | 常州康丽制药有限公司 | Preparation method of 11-(1-piperazinyl) dibenzo[b, f][1,4] thiazepine fumarate |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0240228A1 (en) * | 1986-03-27 | 1987-10-07 | Ici Americas Inc. | Thiazepine compounds |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB8705574D0 (en) * | 1987-03-10 | 1987-04-15 | Ici Plc | Preparation of thiazepine compound |
| GB9716161D0 (en) * | 1997-08-01 | 1997-10-08 | Zeneca Ltd | Process |
| HU227039B1 (en) * | 2000-01-25 | 2010-05-28 | Egis Gyogyszergyar Nyilvanosan | New process for the production of quetiapine and intermediates therefor |
| US7071331B2 (en) * | 2003-02-22 | 2006-07-04 | Teva Pharmaceutical Industries Ltd. | Synthesis of quetiapine and pharmaceutically acceptable salts thereof |
-
2005
- 2005-03-07 ES ES200500513A patent/ES2234447B1/en not_active Expired - Fee Related
- 2005-12-21 US US11/817,884 patent/US7902355B2/en not_active Expired - Fee Related
- 2005-12-21 AT AT05850361T patent/ATE467624T1/en active
- 2005-12-21 EP EP05850361A patent/EP1856073B1/en active Active
- 2005-12-21 DE DE602005021256T patent/DE602005021256D1/en active Active
- 2005-12-21 PT PT05850361T patent/PT1856073E/en unknown
- 2005-12-21 WO PCT/EP2005/014055 patent/WO2006094549A1/en not_active Application Discontinuation
- 2005-12-21 DK DK05850361.6T patent/DK1856073T3/en active
-
2007
- 2007-10-08 NO NO20075071A patent/NO20075071L/en not_active Application Discontinuation
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0240228A1 (en) * | 1986-03-27 | 1987-10-07 | Ici Americas Inc. | Thiazepine compounds |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7687622B2 (en) | 2005-04-14 | 2010-03-30 | Teva Pharmaceutical Industries, Ltd | Process for preparing quetiapine fumarate |
| CZ300451B6 (en) * | 2006-07-03 | 2009-05-20 | Farmak, A. S. | Process for preparing salts of 2-[2-(4-dibenzo[b,f][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]ethanol (quetiapine) and purification process of such salts |
| JP2011510960A (en) * | 2008-01-31 | 2011-04-07 | フェルミオン オサケ ユキチュア | Method for producing quetiapine |
| US8420807B2 (en) | 2008-01-31 | 2013-04-16 | Fermion Oy | Process for the preparation of quetiapine |
| WO2010100623A1 (en) | 2009-03-04 | 2010-09-10 | Ranbaxy Laboratories Limited | Process for the preparation of quetiapine fumarate |
| CN103664822A (en) * | 2012-09-18 | 2014-03-26 | 四川大学华西医院 | Compound used as anesthetic |
Also Published As
| Publication number | Publication date |
|---|---|
| ATE467624T1 (en) | 2010-05-15 |
| PT1856073E (en) | 2010-07-06 |
| EP1856073A1 (en) | 2007-11-21 |
| US7902355B2 (en) | 2011-03-08 |
| US20080171869A1 (en) | 2008-07-17 |
| EP1856073B1 (en) | 2010-05-12 |
| ES2234447B1 (en) | 2006-03-01 |
| DE602005021256D1 (en) | 2010-06-24 |
| DK1856073T3 (en) | 2010-08-23 |
| NO20075071L (en) | 2007-11-28 |
| ES2234447A1 (en) | 2005-06-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7902355B2 (en) | Process for the preparation of an 11-(4-substituted-1-piperazinyl)dibenzo[b,f][1,4]thiazepine derivative | |
| KR960007088B1 (en) | Process for the preparation of a thiazepine compound | |
| EP1252151B1 (en) | A process for the preparation of quetiapine and intermediates therefor | |
| WO2003016287A2 (en) | Process for preparing 1-(carboxymethyl)- and 1-(aminocarbonyl)- pyrimidin-4-one derivatives | |
| EP1660469B1 (en) | Process for the preparation of 11-(1-piperazinyl)dibenzo 'b, f! '1 ,4!-thiazepine, an intermediate in the synthesis of the antipsychotic drug quetiapine | |
| EP1841751A1 (en) | INDUSTRIAL PREPARATION OF 11-(4-(2-(2-HYDROXYETHOXY ETHYL)-1-PIPERAZINYL)DIBENZO (b,f)-1(1, 4)THIAZEPINE | |
| EP2245021B1 (en) | A process for the preparation of quetiapine | |
| EP1660468B1 (en) | Procedure for preparing 11-(4-[2-(2-hydroxyethoxy)ethyl]-1-piperazinyl)-dibenzo[b,f][1,4]thiazepine | |
| WO2005028457A1 (en) | Preparation of quetiapine | |
| US5539107A (en) | Method for the production of azaphenothiazines | |
| KR19990081747A (en) | Method for preparing ester of [2- [4-[(4-chlorophenyl) phenylmethyl] -1-piperazinyl] ethoxy] acetic acid | |
| WO2006027789A1 (en) | PROCESS FOR PRODUCING 11-[4-[2-(2-HYDROXYETHOXY)ETHYL]-1-PIPERAZINYL]DIBENZO[b,f][1,4]THIAZEPINE AND A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF | |
| CA2150948A1 (en) | Process for the preparation of alpha-aryl-gamma-butyrolactones | |
| EP1468992B1 (en) | A process for the preparation of opipramol | |
| EP2297122B1 (en) | New process for the preparation of levocetirizine and intermediates thereof | |
| EP0430012B1 (en) | Process for preparing 2-Aromatic-3-halobenzothiazepines | |
| WO2010085976A1 (en) | Process for the synthesis of quetiapine |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2005850361 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 11817884 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| NENP | Non-entry into the national phase |
Ref country code: RU |
|
| WWW | Wipo information: withdrawn in national office |
Country of ref document: RU |
|
| WWP | Wipo information: published in national office |
Ref document number: 2005850361 Country of ref document: EP |