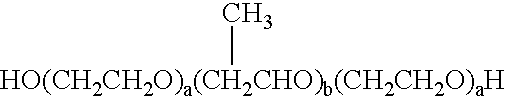US20030055067A1 - Antifungal composition with enhanced bioavailability - Google Patents
Antifungal composition with enhanced bioavailability Download PDFInfo
- Publication number
- US20030055067A1 US20030055067A1 US10/114,612 US11461202A US2003055067A1 US 20030055067 A1 US20030055067 A1 US 20030055067A1 US 11461202 A US11461202 A US 11461202A US 2003055067 A1 US2003055067 A1 US 2003055067A1
- Authority
- US
- United States
- Prior art keywords
- liquid
- suspension
- sorbitan
- effective amount
- liquid suspension
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- LBCPPUVRLUPWLK-JGQPESNZSA-N *.CCC([C@@H](C)O)N1N=CN(C2=CC=C(C)C=C2)C1=O.S.S.[H][C@@]1(COC2=CC=C(N3CCN(C)CC3)C=C2)CO[C@@](CN2C=NC=N2)(C2=C(F)C=C(F)C=C2)C1 Chemical compound *.CCC([C@@H](C)O)N1N=CN(C2=CC=C(C)C=C2)C1=O.S.S.[H][C@@]1(COC2=CC=C(N3CCN(C)CC3)C=C2)CO[C@@](CN2C=NC=N2)(C2=C(F)C=C(F)C=C2)C1 LBCPPUVRLUPWLK-JGQPESNZSA-N 0.000 description 9
- LCCHLJHAGWAXAV-JVEMOQCWSA-N *.S.S.[H][C@@]1(COC2=CC=C(N3CCN(C4=CC=C(N5C=NN(C(CC)[C@@H](C)O)C5=O)C=C4)CC3)C=C2)CO[C@@](CN2C=NC=N2)(C2=C(F)C=C(F)C=C2)C1 Chemical compound *.S.S.[H][C@@]1(COC2=CC=C(N3CCN(C4=CC=C(N5C=NN(C(CC)[C@@H](C)O)C5=O)C=C4)CC3)C=C2)CO[C@@](CN2C=NC=N2)(C2=C(F)C=C(F)C=C2)C1 LCCHLJHAGWAXAV-JVEMOQCWSA-N 0.000 description 4
- OTMSDBZUPAUEDD-UHFFFAOYSA-N CC Chemical compound CC OTMSDBZUPAUEDD-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/64—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with three nitrogen atoms as the only ring hetero atoms
- A01N43/647—Triazoles; Hydrogenated triazoles
- A01N43/653—1,2,4-Triazoles; Hydrogenated 1,2,4-triazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0087—Galenical forms not covered by A61K9/02 - A61K9/7023
- A61K9/0095—Drinks; Beverages; Syrups; Compositions for reconstitution thereof, e.g. powders or tablets to be dispersed in a glass of water; Veterinary drenches
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/10—Antimycotics
Definitions
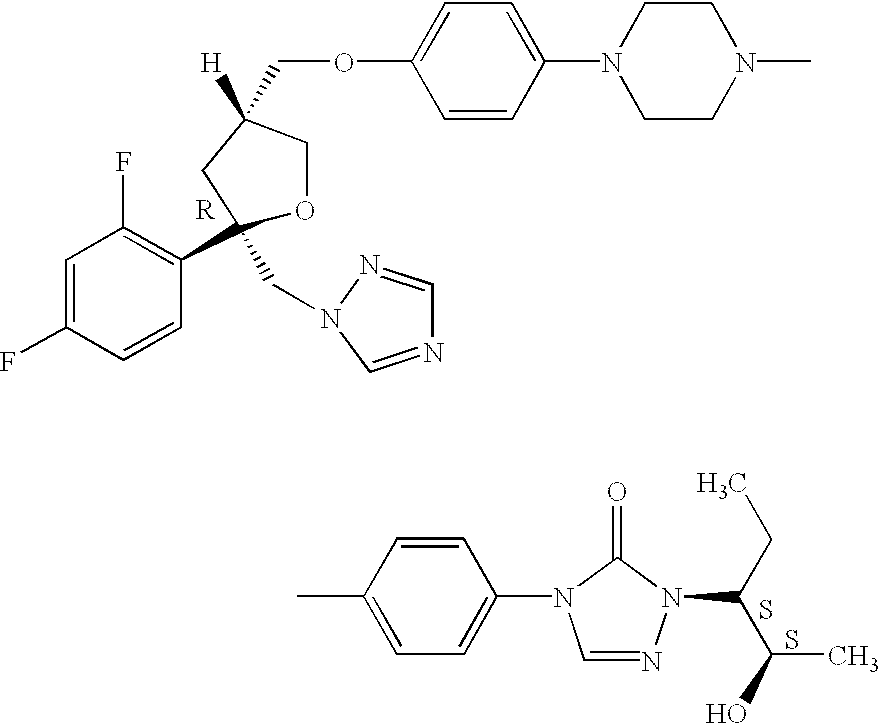
- This invention relates to stable, liquid suspensions containing an antifungally effective amount of the micronized compound represented by the chemical structural formula I:
- At least one thickening agent, a non-ionic surfactant, and a pharmaceutically acceptable liquid carrier at least one thickening agent, a non-ionic surfactant, and a pharmaceutically acceptable liquid carrier, and methods of using the suspensions to treat or prevent fungal infections
- U.S. Pat. No. 5,661,151 discloses the compound of formula I and its potent antifungal activity against a broad range of fungi such as Aspergillis, Candida, Cryptococcus, Fusarium, and other opportunistic fungi.
- U.S. Pat. Nos. 5,834,472 and 5,846,971 disclose oral pharmaceutical capsule compositions of the compound of structural formula I coated onto inert beads together with a binder.
- the compound of structural formula I is highly lipophilic, and has an extremely low water solubility.
- aqueous compositions of the compound of structural formula I were found to have reduced anti-fungal activity and/or bioavailability, presumably due to the extremely low water solubility of the compound. Accordingly, a need exists for an oral pharmaceutical composition of the compound of structural formula I that has enhanced bioavailability and improved stability characteristics
- compositions in the form of liquid suspension, suitable for oral administration comprising micronized particles of posaconazole, the compound having the chemical structural formula I
- At least one thickening agent, a non-ionic surfactant, and a pharmaceutically acceptable liquid carrier that provides significant advantages over the prior art.
- Advantages of the liquid suspensions of the present invention include improved homogeneity of the suspension and ease of dispersibility of the suspension.
- the solids that settle in the liquid suspension of the present invention do not form a solid cake that is difficult to re-disperse. There is virtually no sedimentation of the solids in the unreconstituted liquid suspension of this invention for a period of at least three days.
- This surprising feature ensures that a patient having a fungal infection taking the liquid suspensions of the present invention will receive an antifungally effective amount of posaconazole.
- the liquid suspensions of the present invention have a longer shelf life. Additionally, the liquid suspension, upon reconstitution, provide substantially the same antifungally effective amount posaconazole as the initially prepared suspension.
- the present invention provides a liquid suspension comprising micronized posaconazole, having the chemical structural formula I:
- At least one thickening agent at least one thickening agent, a non-ionic surfactant, and a pharmaceutically acceptable liquid carrier.
- This invention also provides a liquid suspension comprising an antifungally effective amount of micronized posaconazole having the chemical structural formula I:
- an effective amount of at least one thickening agent an amount of a buffer system effective to maintain the pH of the system in the range of about 4.0 to about 6.0, an effective amount of a non-ionic surfactant, and a pharmaceutically acceptable liquid carrier.
- This invention further provides a liquid suspension comprising an antifungally effective amount of micronized posaconazole having the chemical structural formula I:
- the micronized compound has a mean particle size in the range of about 1200 nm to about 1600 nm, an effective amount of polyoxyethylene derivatives of sorbitan esters of saturated or unsaturated C 12 to C 18 acids, an effective amount of a buffer system sufficient to maintain a pH in the range of about 4.0 to about 6.0, an effective amount of a combination of two thickening agents, wherein one is a liquid sugar, and a pharmaceutically acceptable liquid carrier.
- FIGS. I & 2 graphically display the mean plasma concentration time profiles of posaconazole tablets and of the liquid suspension of Example 1 of the present invention.
- FIG. 1 is a linear:linear graphic profile of the plasma concentration (ng/ml)of the compound of formula I versus time (hours) after administration of the following four Treatments A- D: a single of 2 ⁇ 100 mg of the compound of formula I in the tablet co-precipitate formulation of U.S. Pat. No. 5,834,472 with a standardized high-fat breakfast- Treatment D and. symbol -•-; a 200 mg of the compound of formula I in the oral suspension of this invention (5 ml) following a 10-hr. fast -Treatment A and.
- FIG. 2 is a log linear graphic profile of the plasma concentration (ng/ml)of the compound of formula I versus time in hours for the data presented in FIG. 1.
- the present invention provides a stable suspension of micronized particles of the antifungal compound posaconazole in a pharmaceutically acceptable liquid carrier.
- the suspension of the present invention is stable to settling without sedimentation when stored undisturbed for more than three days at 25° C. (See Table 1 below).
- Table 2 below shows that the liquid suspension formulations of this invention are stable in that the concentration of posaconazole in the suspension is substantially the same ( ⁇ 2%) compared to the initial concentration (as measured by HPLC) for periods of up to 12 months.
- the stable suspension of the present invention has a remarkably higher (23-36% increase) bioavailability compared to an optimized oral tablet of micronized particles of posaconazole when each is administered to subjects concurrently with a high fat breakfast. See Tables 3 and 4 and FIGS. 1 & 2
- One aspect of the present invention is to provide a pharmaceutical composition that contains micronized particles of posaconazole in combination with a non-ionic surfactant, such as a sorbitan ester and at least one thickening agent, preferably a combination of xanthan gum and a liquid sugar, that are easily dispersible in a pharmaceutically acceptable liquid carrier such as purified water.
- a pharmaceutically acceptable liquid carrier such as purified water.
- the pharmaceutical composition provides a stabilized suspension that does not settle for at least three days, thus ensuring that patients will get an effective dose of posaconazole.
- Another feature of the stabilized suspension of the present invention is that it is useful in treating patients with HIV-1 infections with oral thrush without posaconazole precipitating out of solution.
- Another aspect of the present invention is that the suspension of the present invention avoids formation of solid cakes which are difficult to disperse.
- Micron-sized particles of posaconazole preferably have a mean particle size range of about 1000 nanometers (nm) to about 1800 nm, preferably about 1200 nm to about 1600 nm, and most preferably about 1400 nm. This particle size can be obtained either by the final step during the manufacture of the antifungal compound of formula I or by the use of conventional micronizing techniques after the conventional crystallization procedure(s).
- microfluidization is an alternative to traditional homogenization that utilizes the collision of two product streams at high pressures to produce a much more uniform particle size distribution (according to Microfluidics International Co.) and smaller average particle size of about 1200 nm to 1600 nm.
- the process and equipment used in microfluidization are described in U.S. Pat. No. 4,533,254.
- the micronized posaconazole of the present invention may also be present in crystalline form. It is preferably substantially chemically and optically pure, and it contains less than about 10% of its optical isomers, enantiomers or other diastereomers. It may be 99% of the optically pure the levorotatory or dextrarotatory isomer. This optically pure compound of chemical structure I should avoid many of the untoward side effects of a mixture of other optical isomers.
- Posaconazole liquid suspension is employed in the composition in antifungally amounts effective to control the fungi of interest.
- antifungally effective amounts can range from about 10 mg/ml to about 100 mg/ml concentration of the liquid suspension formulations of the present invention, more preferably from about 20 mg/ml to about 60 mg/ml, and most preferably about 40 mg/ml of the compound of formula I.
- the present invention also provides for a method of treating and/or preventing fungal infection in a mammal comprising administering to the mammal an amount of the liquid suspension containing the micronized posaconazole in an amount effective for treating and/or preventing such fungal infection.
- Antifungally effective amounts of liquid suspensions of present invention containing 40 mg/ml of the compound of formula I is administered orally in the doses of 5 ml—containing 200 mg of formula I—three times a day (TID) or four times a day (QID)—or 10 ml—containing 400 mg of the compound of formula 1—twice a day (BID).
- TID three times a day
- QID four times a day
- 10 ml containing 400 mg of the compound of formula 1—twice a day
- BID twice a day
- the attending clinician may change the dose and dosing regimen in view of the age, health, and sex of the patient as well as the severity of the fungal infection.
- Non-ionic surfactant refers to a surfactant which lacks a net ionic charge and does not dissociate to an appreciable extent in aqueous media.
- the properties of non-ionic surfactants are largely dependent upon the proportions of the hydrophilic and hydrophobic groups in the molecule.
- Hydrophilic groups include the oxyethylene group (—OCH 2 CH 2 —) and the hydroxy group. By varying the number of these groups in a hydrophobic molecule, such as an ester of a fatty acid, substances are obtained which range from strongly hydrophobic and water insoluble compounds, such as glyceryl monostearate, to strongly hydrophilic and water-soluble compounds, such as the macrogols.
- hydrophilic and hydrophobic groups are more evenly balanced, such as the macrogol esters and ethers and sorbitan derivatives.
- Suitable non-ionic surfactants may be found in Martindale, The Extra Pharmacopoeia, 28th Edition, 1982, The Pharmaceutical Press, London, Great Britain, pp. 370 to 379.
- Such suitable non-ionic surfactants include block copolymers of ethylene oxide and propylene oxide, glycol or glyceryl esters of saturated or unsaturated C 8 to C 20 acids, preferably, polyoxyethylene esters of saturated or unsaturated C 8 to C 20 acids, polyoxyethylene ethers of saturated or unsaturated C 8 to C 20 acids, and polyvinylalcohols or sorbitan esters of saturated or unsaturated C 10 to C 20 acids.
- the non-ionic surfactant is a sorbitan ester of a saturated or unsaturated C 10 to C 20 acid, and more preferably the sorbitan ester is a fatty acid ester of sorbitan selected from sorbitan monolaurate, sorbitan monooleate, sorbitan sesquioleate, sorbitan trioleate, sorbitan monopalmitate, sorbitan monostearate and sorbitan tristearate, or mixtures thereof.
- Suitable sorbitan esters include, e.g. Polysorbate 20, Polysorbate 40, Polysorbate 60, Polysorbate 65, Polysorbate 80, Polysorbate 85, Sorbitan Monolaurate, Sorbitan Mono-oleate, Sorbitan Monopalmitate, Sorbitan Monostearate, Sorbitan Sesquioleate, Sorbitan Trioleate and Sorbitan Tristearate.
- the most preferred non-ionic surfactant is Polysorbate 80, available from ICI Americas under the tradename Tween 80 which is a mixture of oleate esters of sorbitol and sorbitol anhydrides, consisting predominantly of the monoester, condensed with approximately 20 moles of ethylene oxide.
- Suitable block copolymers of ethylene oxide and propylene oxide generically called “Poloxamers” and include those represented by the following chemical structure I:
- a is an integer ranging from about 10 to about 110, preferably from about 12 to 101; more preferably from about 12 to 80; and
- b is an integer ranging from about 20 to about 60, more preferably from about 20 to about 56; also from about 20 to 27.
- Suitable glycol and glyceryl esters of fatty acids include glyceryl monooleate and similar water soluble derivatives
- Suitable polyoxyethylene esters of fatty acids include polyoxyethylene castor oil and hydrogenated castor oil derivatives;
- Suitable polyoxyethylene ethers of fatty acids include Cetomacrogel 1000, Lauromacrogols (a series of lauryl ethers of macrogols of differing chain lengths), e.g. Laureth 4 , Laureth 9 and Lauromacrogol 400.
- the effective amount of non-ionic surfactant in the composition can range from about 5 mg/ml to about 50 mg/ml concentration of the formulation, more preferably from about 5 mg/ml to about 25 mg/ml, and most preferably 10 mg/ml.
- Thickening agents found suitable in the present invention include any commercially available agent useful for such purpose.
- Xanthan gum, liquid sugars, starches, celluloses and mixtures thereof are preferred thickening agents. More preferred is a combination of xanthan gum and a liquid sugar and, most preferred is a combination of xanthan gum, NF and glucose, NF.
- the xanthan gum is present in an amount of about 1 mg/ml to about 5 mg/ml, and more preferably the xanthan gum is present in an amount of about 3 mg/ml.
- the glucose NF is present in an amount of about 200 to about 500 mg/ml, and more preferably about 350 mg/ml.
- the effective amount of thickening agent of the present invention may be about 1 to about 500 mg/ml, more preferably about 200 to about 500 mg/ml, most preferably about 353 mg/ml.
- the thickening agents of the present invention facilitate suspension of the formulation after constitution with minimum agitation and prevent rapid settling and caking of the suspension over time.
- Pharmaceutically acceptable carriers include those well known in the art, including purified water USP, liquid glucose, NF, and anhydrous glycerol. Most preferred is purified water USP and liquid glucose, NF.
- the pharmaceutically acceptable carrier may be present in an amount of about 10 to about 500 mg/ml, more preferably about 200 mg/ml to about 400 mg/ml, most preferably about 350 mg/ml.
- the buffer systems suitable for the formulations of the present invention are those which maintain the pH of the liquid suspension in the range of about 4 to about 6, preferably in the 4.5 to 5.0, and most preferably about 4.5.
- Other suitable buffer systems may be used to maintain the desired pH range of 4 to 6.
- the buffering agents may be present in a concentration of about 0.4 to about 1.5 mg/ml, more preferably about 0.7 to about 1.5 mg/ml, most preferably about 1.1 mg/ml.
- Anti-foaming agents found suitable in the present invention include any commercially available agent useful for such purpose including the methylated linear siloxsane polymers end blocked with trimethylsiloxyl units such as dimethicone and simethicone, as well as mixtures of dimethicone with an average chain length of 200 to 250 dimethylsiloxane units and silica gel.
- the effective amount of anti-foaming agents is an amount sufficient to provide a concentration of about 2 mg/ml to about 4 mg/ml, preferably about 3 mg/ml.
- the water soluble preservatives found useful in present invention include sodium benzoate, sodium citrate and benzalkonium chloride as well as other pharmaceutically acceptable water soluble preservatives.
- Use of sodium benzoate as a preservative is preferred.
- the effective amount of the water soluble preservative is an amount sufficient to provide a concentration of about 0.5 mg/ml to about 3 mg/ml, most preferably about 2 mg/ml.
- the opacifier agents found suitable in the present invention include pharmaceutically acceptable metal oxides, especially titanium dioxide.
- the effective amount of the opacifier agent is an amount sufficient to provide a concentration of about 2 mg/ml to about 6 mg/ml, most preferably about 4 mg/ml.
- Typical flavoring agents are those that are approved by FDA for use in sweetened pharmaceuticals, foods, candies, beverages and the like; these materials impart flavors such as grape, cherry, citrus, peach, strawberry, bubble gum, peppermint and many others.
- the effective amount of the flavoring agents is an amount sufficient to provide a concentration of about 0.01 mg/ml to about 6 mg/ml, more preferably about 5 mg/ml.
- compositions of the present invention containing posaconazole are not to be interpreted as limiting the scope of the claims.
- Concentration Range Ingredient (mg/ml) Posaconazole (micronized) 10-100 Polysorbate 80 5-50 Sodium Citrate, USP, 0.4-0.8 Monohydrate Citric Acid, USP, Monohydrate 0.36-0.6 Simethicone, USP 2-4 Xanthan Gum, NF 1-5 Sodium Benzoate, NF 0.5-3 Liquid Glucose, NF 200-500 Glycerin, USP 50-150 Artificial Cherry Flavor 2-10 Titanium Dioxide 2-6 Purified Water, USP q.s. ad —
- Concentration Ingredient (mg/ml) Posaconazole (micronized) 40 Polysorbate 80 10 Sodium Citrate, USP, 0.6 Monohydrate Citric Acid, USP, Monohydrate 0.48 Simethicone, USP 3 Xanthan Gum, NF 3 Sodium Benzoate, NF 2 Liquid Glucose, NF 350 Glycerin, USP 100 Artificial Cherry Flavor 5 Titanium Dioxide 4 Purified Water, USP q.s. ad 1 ml
- This example is prepared as follows: charge approximately 5% of the final batch volume of purified water at 20 ⁇ 3° C. to a suitable vessel equipped with a mixer propeller. Add 40% of the polysorbate 80 to the purified water in step 1 and mix until dissolved. Add 40% of the simethicone and mix until it is dispersed. Recirculate the mixture in step 3 through a Microfluidizer, operating at about 30,000 ⁇ 5000 psi for approximately 5 passes. Add approximately 7% of the final batch volume of purified water at about 20 ⁇ 3° C., and mix for approximately five minutes. Add the Posaconazole to the vessel in step 5 with constant mixing. Continue mixing until it is fully dispersed.
- Step 6 Recirculate the suspension portion from step 6 through a Microfluidizer, operating at a pressure of about 30,000 ⁇ 5,000 psi. Process the concentrate until the median of the particles shows a particle size of about 1.4 ⁇ 0.2 ⁇ m, when measured via laser diffraction techniques that are known in the art.
- Example 2 is another example of a formulation within the scope of the present invention prepared using the procedure of Example 1 and has a pH of 4.5.
- Concentration Ingredient (mg/ml) Posaconazole (micronized) 40 Polysorbate 80 10 Sodium Citrate, USP, 0.6 Monohydrate Citric Acid, USP, Monohydrate 1.5 Simethicone, USP 3 Xanthan Gum, NF 3 Sodium Benzoate, NF 2 Liquid Glucose, NF 350 Glycerin, USP 100 Artificial Cherry Flavor 5 Titanium Dioxide 4 Purified Water, USP q.s. ad 1 ml
- the sodium benzoate was not expected to sediment. Surprisingly, the posaconazole was not sedimented after 3 days.
- compositions of the present invention have both ease of dispersibility and homogeneity as is evidenced by the stability of the samples in Table 1.
- Bioavailability is defined as the rate and extent to that the active drug ingredient or therapeutic moiety is absorbed into the systemic circulation from an administered dosage form as compared to a standard or control.
- C max value is defined as the maximum concentration of the antifungal compound measured (i.e. “peak”) in the plasma serum.
- Formulations of the present invention have the advantage that they have an increased bioavailability and lower variability than previous formulations.
- the first objective was to determine the relative bioavailability of posaconazole given as an oral suspension compared to an oral solid formulation when administered with a high-fat breakfast.
- the second objective was to determine the effect of high-fat and non-fat food relative to fasting conditions on the oral bioavailability of the compound of formula I when given as an oral suspension.
- Treatment A 200 mg of the compound of formula I in the oral suspension of this invention (5 ml) following a 10-hr.
- fast Treatment B 200 mg of the compound of formula I in the oral suspension of this invention (5 ml) with a standardized high-fat breakfast
- Treatment C 200 mg of the compound of formula I in the oral suspension of this invention (5 ml) with a standardized non-fat breakfast
- Treatment D 2 ⁇ 100 mg of the compound of formula I in the tablets (co-precipitate formulation of U.S. Pat. No. 5,834,472 with a standardized high-fat breakfast
- Subjects were randomized to either remain fasted (Treatment A), to receive a standardized high fat breakfast (Treatment B or D) or a standardized non-fat breakfast (Treatment C). Meals were consumed in a 20-minute period prior to the morning drug administration and subjects received the appropriate treatment within 5 minute of completing the breakfast.
- Blood samples (6 ml) were collected into heparinized tubes for each treatment immediately prior to dosing (0 hour) and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 24, 36, 48 and 72 hours after dosing. Blood was centrifuged at 4° C. and plasma stored at or below ⁇ 20° C. until assayed. Plasma concentrations of posaconazole were assayed using a validated high performance liquid chromatographic assay with a LOQ of ng/ml.
- C(tf) is the estimated concentration determined from linear regression at time, tf.
- Vdarea/F The apparent volume of distribution (Vdarea/F) was calculated from the ratio of the total body clearance to the terminal phase rate constant (K).
- suspension formulation of the present invention resulted in a 23% increase in Cmax and a 36% increase in AUC(tf) compared to the tablet of the prior art.
- the secondary objective of the study was to evaluate the effect of high fat food (Treatment B) and non-fat food (Treatment C) compared to fasting (Treatment A) on the oral bioavailability of posaconazole administered as an oral suspension.
- a high fat breakfast produced a 4-fold increase in the bioavailability of posaconazole given in a suspension. This was consistent with results from a previous study where food significantly increased the bioavailability of posaconazole by 3-5-fold for both tablet and capsule formulations. The effect of a non-fat breakfast (Treatment C) compared to fasting (Treatment A) was less, with a 2.5-3-fold increase in bioavailability.
Abstract
Description
-
- at least one thickening agent, a non-ionic surfactant, and a pharmaceutically acceptable liquid carrier, and methods of using the suspensions to treat or prevent fungal infections
- U.S. Pat. No. 5,661,151 discloses the compound of formula I and its potent antifungal activity against a broad range of fungi such as Aspergillis, Candida, Cryptococcus, Fusarium, and other opportunistic fungi.
- U.S. Pat. Nos. 5,834,472 and 5,846,971, disclose oral pharmaceutical capsule compositions of the compound of structural formula I coated onto inert beads together with a binder. However, the compound of structural formula I is highly lipophilic, and has an extremely low water solubility. Thus, aqueous compositions of the compound of structural formula I were found to have reduced anti-fungal activity and/or bioavailability, presumably due to the extremely low water solubility of the compound. Accordingly, a need exists for an oral pharmaceutical composition of the compound of structural formula I that has enhanced bioavailability and improved stability characteristics
-
- at least one thickening agent, a non-ionic surfactant, and a pharmaceutically acceptable liquid carrier that provides significant advantages over the prior art.
- Advantages of the liquid suspensions of the present invention include improved homogeneity of the suspension and ease of dispersibility of the suspension. The solids that settle in the liquid suspension of the present invention do not form a solid cake that is difficult to re-disperse. There is virtually no sedimentation of the solids in the unreconstituted liquid suspension of this invention for a period of at least three days. This surprising feature ensures that a patient having a fungal infection taking the liquid suspensions of the present invention will receive an antifungally effective amount of posaconazole. The liquid suspensions of the present invention have a longer shelf life. Additionally, the liquid suspension, upon reconstitution, provide substantially the same antifungally effective amount posaconazole as the initially prepared suspension. These features of the liquid suspensions of the present invention provide benefits to pharmacies, pharmacists, doctors and patients having fungal infections.
-
- at least one thickening agent, a non-ionic surfactant, and a pharmaceutically acceptable liquid carrier.
-
- an effective amount of at least one thickening agent, an amount of a buffer system effective to maintain the pH of the system in the range of about 4.0 to about 6.0, an effective amount of a non-ionic surfactant, and a pharmaceutically acceptable liquid carrier.
-
- wherein the micronized compound has a mean particle size in the range of about 1200 nm to about 1600 nm, an effective amount of polyoxyethylene derivatives of sorbitan esters of saturated or unsaturated C 12 to C18 acids, an effective amount of a buffer system sufficient to maintain a pH in the range of about 4.0 to about 6.0, an effective amount of a combination of two thickening agents, wherein one is a liquid sugar, and a pharmaceutically acceptable liquid carrier.
- FIGS. I & 2 graphically display the mean plasma concentration time profiles of posaconazole tablets and of the liquid suspension of Example 1 of the present invention. FIG. 1 is a linear:linear graphic profile of the plasma concentration (ng/ml)of the compound of formula I versus time (hours) after administration of the following four Treatments A- D: a single of 2×100 mg of the compound of formula I in the tablet co-precipitate formulation of U.S. Pat. No. 5,834,472 with a standardized high-fat breakfast- Treatment D and. symbol -•-; a 200 mg of the compound of formula I in the oral suspension of this invention (5 ml) following a 10-hr. fast -Treatment A and. symbol -O-;a 200 mg of the compound of formula I in the oral suspension of this invention (5 ml) with a standardized high-fat breakfast-Treatment B and. symbol -Δ-;and a 200 mg of the compound of formula I in the oral suspension of this invention (5 ml) with a standardized non-fat breakfast-Treatment C and. symbol-□-.
- FIG. 2 is a log linear graphic profile of the plasma concentration (ng/ml)of the compound of formula I versus time in hours for the data presented in FIG. 1.
- The present invention provides a stable suspension of micronized particles of the antifungal compound posaconazole in a pharmaceutically acceptable liquid carrier. The suspension of the present invention is stable to settling without sedimentation when stored undisturbed for more than three days at 25° C. (See Table 1 below).
- Table 2 below shows that the liquid suspension formulations of this invention are stable in that the concentration of posaconazole in the suspension is substantially the same (±2%) compared to the initial concentration (as measured by HPLC) for periods of up to 12 months.
- We have also found that the stable suspension of the present invention has a remarkably higher (23-36% increase) bioavailability compared to an optimized oral tablet of micronized particles of posaconazole when each is administered to subjects concurrently with a high fat breakfast. See Tables 3 and 4 and FIGS. 1 & 2
- One aspect of the present invention is to provide a pharmaceutical composition that contains micronized particles of posaconazole in combination with a non-ionic surfactant, such as a sorbitan ester and at least one thickening agent, preferably a combination of xanthan gum and a liquid sugar, that are easily dispersible in a pharmaceutically acceptable liquid carrier such as purified water. The pharmaceutical composition provides a stabilized suspension that does not settle for at least three days, thus ensuring that patients will get an effective dose of posaconazole. Another feature of the stabilized suspension of the present invention is that it is useful in treating patients with HIV-1 infections with oral thrush without posaconazole precipitating out of solution. Another aspect of the present invention is that the suspension of the present invention avoids formation of solid cakes which are difficult to disperse.
- The compound of formula I used in the suspensions of the present invention is available from Schering Corporation, Kenilworth, N.J., and has been prepared according to Examples 24 and 32 of U.S. Pat. No. 5,661,151 and WO 95/17407.
- Micron-sized particles of posaconazole preferably have a mean particle size range of about 1000 nanometers (nm) to about 1800 nm, preferably about 1200 nm to about 1600 nm, and most preferably about 1400 nm. This particle size can be obtained either by the final step during the manufacture of the antifungal compound of formula I or by the use of conventional micronizing techniques after the conventional crystallization procedure(s).
- The preferred micronizing technique that is employed to micronize the posaconazole to the desired mean particle size range is microfluidization. Microfluidization is an alternative to traditional homogenization that utilizes the collision of two product streams at high pressures to produce a much more uniform particle size distribution (according to Microfluidics International Co.) and smaller average particle size of about 1200 nm to 1600 nm. The process and equipment used in microfluidization are described in U.S. Pat. No. 4,533,254.
- The micronized posaconazole of the present invention may also be present in crystalline form. It is preferably substantially chemically and optically pure, and it contains less than about 10% of its optical isomers, enantiomers or other diastereomers. It may be 99% of the optically pure the levorotatory or dextrarotatory isomer. This optically pure compound of chemical structure I should avoid many of the untoward side effects of a mixture of other optical isomers.
- Posaconazole liquid suspension is employed in the composition in antifungally amounts effective to control the fungi of interest. Such antifungally effective amounts can range from about 10 mg/ml to about 100 mg/ml concentration of the liquid suspension formulations of the present invention, more preferably from about 20 mg/ml to about 60 mg/ml, and most preferably about 40 mg/ml of the compound of formula I.
- The present invention also provides for a method of treating and/or preventing fungal infection in a mammal comprising administering to the mammal an amount of the liquid suspension containing the micronized posaconazole in an amount effective for treating and/or preventing such fungal infection. Antifungally effective amounts of liquid suspensions of present invention containing 40 mg/ml of the compound of formula I is administered orally in the doses of 5 ml—containing 200 mg of formula I—three times a day (TID) or four times a day (QID)—or 10 ml—containing 400 mg of the compound of formula 1—twice a day (BID). Of course, the attending clinician may change the dose and dosing regimen in view of the age, health, and sex of the patient as well as the severity of the fungal infection.
- The following terms are used to describe the present pharmaceutical compositions, ingredients that may be employed in its formulation and methods for assessing the compound's bioactivity or bioavailability.
- Non-ionic surfactant refers to a surfactant which lacks a net ionic charge and does not dissociate to an appreciable extent in aqueous media. The properties of non-ionic surfactants are largely dependent upon the proportions of the hydrophilic and hydrophobic groups in the molecule. Hydrophilic groups include the oxyethylene group (—OCH 2CH2—) and the hydroxy group. By varying the number of these groups in a hydrophobic molecule, such as an ester of a fatty acid, substances are obtained which range from strongly hydrophobic and water insoluble compounds, such as glyceryl monostearate, to strongly hydrophilic and water-soluble compounds, such as the macrogols. Between these two extremes types include those in which the proportions of the hydrophilic and hydrophobic groups are more evenly balanced, such as the macrogol esters and ethers and sorbitan derivatives. Suitable non-ionic surfactants may be found in Martindale, The Extra Pharmacopoeia, 28th Edition, 1982, The Pharmaceutical Press, London, Great Britain, pp. 370 to 379.
- Such suitable non-ionic surfactants include block copolymers of ethylene oxide and propylene oxide, glycol or glyceryl esters of saturated or unsaturated C 8 to C20 acids, preferably, polyoxyethylene esters of saturated or unsaturated C8 to C20 acids, polyoxyethylene ethers of saturated or unsaturated C8to C20 acids, and polyvinylalcohols or sorbitan esters of saturated or unsaturated C10to C20 acids. Preferably, the non-ionic surfactant is a sorbitan ester of a saturated or unsaturated C10 to C20 acid, and more preferably the sorbitan ester is a fatty acid ester of sorbitan selected from sorbitan monolaurate, sorbitan monooleate, sorbitan sesquioleate, sorbitan trioleate, sorbitan monopalmitate, sorbitan monostearate and sorbitan tristearate, or mixtures thereof.
- Suitable sorbitan esters include, e.g. Polysorbate 20, Polysorbate 40,
Polysorbate 60, Polysorbate 65, Polysorbate 80, Polysorbate 85, Sorbitan Monolaurate, Sorbitan Mono-oleate, Sorbitan Monopalmitate, Sorbitan Monostearate, Sorbitan Sesquioleate, Sorbitan Trioleate and Sorbitan Tristearate. The most preferred non-ionic surfactant is Polysorbate 80, available from ICI Americas under the tradename Tween 80 which is a mixture of oleate esters of sorbitol and sorbitol anhydrides, consisting predominantly of the monoester, condensed with approximately 20 moles of ethylene oxide. -
- wherein a is an integer ranging from about 10 to about 110, preferably from about 12 to 101; more preferably from about 12 to 80; and
- b is an integer ranging from about 20 to about 60, more preferably from about 20 to about 56; also from about 20 to 27.
- Suitable glycol and glyceryl esters of fatty acids include glyceryl monooleate and similar water soluble derivatives;
- Suitable polyoxyethylene esters of fatty acids (macrogol esters) include polyoxyethylene castor oil and hydrogenated castor oil derivatives;
- Suitable polyoxyethylene ethers of fatty acids (macrogol ethers) include
Cetomacrogel 1000, Lauromacrogols (a series of lauryl ethers of macrogols of differing chain lengths), e.g. Laureth 4, Laureth 9 and Lauromacrogol 400. - The effective amount of non-ionic surfactant in the composition can range from about 5 mg/ml to about 50 mg/ml concentration of the formulation, more preferably from about 5 mg/ml to about 25 mg/ml, and most preferably 10 mg/ml.
- Thickening agents found suitable in the present invention include any commercially available agent useful for such purpose. Xanthan gum, liquid sugars, starches, celluloses and mixtures thereof are preferred thickening agents. More preferred is a combination of xanthan gum and a liquid sugar and, most preferred is a combination of xanthan gum, NF and glucose, NF. Preferably, the xanthan gum is present in an amount of about 1 mg/ml to about 5 mg/ml, and more preferably the xanthan gum is present in an amount of about 3 mg/ml. Preferably, the glucose NF is present in an amount of about 200 to about 500 mg/ml, and more preferably about 350 mg/ml. The effective amount of thickening agent of the present invention may be about 1 to about 500 mg/ml, more preferably about 200 to about 500 mg/ml, most preferably about 353 mg/ml. The thickening agents of the present invention facilitate suspension of the formulation after constitution with minimum agitation and prevent rapid settling and caking of the suspension over time.
- Pharmaceutically acceptable carriers include those well known in the art, including purified water USP, liquid glucose, NF, and anhydrous glycerol. Most preferred is purified water USP and liquid glucose, NF. The pharmaceutically acceptable carrier may be present in an amount of about 10 to about 500 mg/ml, more preferably about 200 mg/ml to about 400 mg/ml, most preferably about 350 mg/ml.
- The buffer systems suitable for the formulations of the present invention are those which maintain the pH of the liquid suspension in the range of about 4 to about 6, preferably in the 4.5 to 5.0, and most preferably about 4.5. The use of a buffer system of sodium citrate, USP and citric acid, USP, is preferred. Other suitable buffer systems may be used to maintain the desired pH range of 4 to 6. The buffering agents may be present in a concentration of about 0.4 to about 1.5 mg/ml, more preferably about 0.7 to about 1.5 mg/ml, most preferably about 1.1 mg/ml.
- Anti-foaming agents found suitable in the present invention include any commercially available agent useful for such purpose including the methylated linear siloxsane polymers end blocked with trimethylsiloxyl units such as dimethicone and simethicone, as well as mixtures of dimethicone with an average chain length of 200 to 250 dimethylsiloxane units and silica gel. The effective amount of anti-foaming agents is an amount sufficient to provide a concentration of about 2 mg/ml to about 4 mg/ml, preferably about 3 mg/ml.
- The water soluble preservatives found useful in present invention include sodium benzoate, sodium citrate and benzalkonium chloride as well as other pharmaceutically acceptable water soluble preservatives. Use of sodium benzoate as a preservative is preferred. The effective amount of the water soluble preservative is an amount sufficient to provide a concentration of about 0.5 mg/ml to about 3 mg/ml, most preferably about 2 mg/ml.
- The opacifier agents found suitable in the present invention include pharmaceutically acceptable metal oxides, especially titanium dioxide. The effective amount of the opacifier agent is an amount sufficient to provide a concentration of about 2 mg/ml to about 6 mg/ml, most preferably about 4 mg/ml.
- Typical flavoring agents are those that are approved by FDA for use in sweetened pharmaceuticals, foods, candies, beverages and the like; these materials impart flavors such as grape, cherry, citrus, peach, strawberry, bubble gum, peppermint and many others. The effective amount of the flavoring agents is an amount sufficient to provide a concentration of about 0.01 mg/ml to about 6 mg/ml, more preferably about 5 mg/ml.
- The following examples describe compositions of the present invention containing posaconazole, but they are not to be interpreted as limiting the scope of the claims.
Concentration Range Ingredient (mg/ml) Posaconazole (micronized) 10-100 Polysorbate 80 5-50 Sodium Citrate, USP, 0.4-0.8 Monohydrate Citric Acid, USP, Monohydrate 0.36-0.6 Simethicone, USP 2-4 Xanthan Gum, NF 1-5 Sodium Benzoate, NF 0.5-3 Liquid Glucose, NF 200-500 Glycerin, USP 50-150 Artificial Cherry Flavor 2-10 Titanium Dioxide 2-6 Purified Water, USP q.s. ad — - The above ranges of ingredients may be varied as is evident to one skilled in the art.
- Specific examples of a composition within the scope of the invention is set forth below.
-
Concentration Ingredient (mg/ml) Posaconazole (micronized) 40 Polysorbate 80 10 Sodium Citrate, USP, 0.6 Monohydrate Citric Acid, USP, Monohydrate 0.48 Simethicone, USP 3 Xanthan Gum, NF 3 Sodium Benzoate, NF 2 Liquid Glucose, NF 350 Glycerin, USP 100 Artificial Cherry Flavor 5 Titanium Dioxide 4 Purified Water, USP q.s. ad 1 ml - This example is prepared as follows: charge approximately 5% of the final batch volume of purified water at 20±3° C. to a suitable vessel equipped with a mixer propeller. Add 40% of the polysorbate 80 to the purified water in step 1 and mix until dissolved. Add 40% of the simethicone and mix until it is dispersed. Recirculate the mixture in step 3 through a Microfluidizer, operating at about 30,000 ±5000 psi for approximately 5 passes. Add approximately 7% of the final batch volume of purified water at about 20±3° C., and mix for approximately five minutes. Add the Posaconazole to the vessel in step 5 with constant mixing. Continue mixing until it is fully dispersed. Recirculate the suspension portion from step 6 through a Microfluidizer, operating at a pressure of about 30,000±5,000 psi. Process the concentrate until the median of the particles shows a particle size of about 1.4±0.2 μm, when measured via laser diffraction techniques that are known in the art.
- When the particle size has been achieved, pass the suspension through the microfluidizer and collect in a suitable sized vessel. Add approximately 8-10% of the final batch volume of purified water (at 20±3° C.) to the feed vessel, and pass through the microfluidizer operating at approximately 30,000 psi. Collect the rinse in the vessel containing the concentrate. Add approximately 22% of the final batch volume of purified water (20±3° C.) to the vessel with the concentrate, and mix for approximately five (5) minutes. Add the remainder of the polysorbate 80 and simethicone, and mix for approximately five (5) minutes.
- Add the sodium benzoate, sodium citrate and citric acid and mix for approximately five (5) minutes. Add the xanthan gum slowly with constant mixing. Continue to mix after addition of the xanthan gum. Allow the xanthan gum to hydrate without mixing for 30 minutes. Add the glycerin with constant mixing. Add the liquid glucose slowly with constant mixing. Mix for five minutes or until it is dissolved. Add the titanium dioxide and mix using a suitable homogenizer until that ingredient is fully dispersed. Add the artificial cherry flavor, and mix for approximately five minutes. Add purified water at 20±3° C., and qs up to a final volume, and mix until a uniform suspension is attained. Collect approximately 20 ml sample for pH measurement and physical observation of the suspension. The pH of the suspension of Example 1 was 5.0.
- Example 2 is another example of a formulation within the scope of the present invention prepared using the procedure of Example 1 and has a pH of 4.5.
Concentration Ingredient (mg/ml) Posaconazole (micronized) 40 Polysorbate 80 10 Sodium Citrate, USP, 0.6 Monohydrate Citric Acid, USP, Monohydrate 1.5 Simethicone, USP 3 Xanthan Gum, NF 3 Sodium Benzoate, NF 2 Liquid Glucose, NF 350 Glycerin, USP 100 Artificial Cherry Flavor 5 Titanium Dioxide 4 Purified Water, USP q.s. ad 1 ml - The rate of sedimentation of the liquid suspension of the present invention was determined as set forth below.
TABLE 1 Posaconazole Oral Suspension 40 mg/ml Rate of Sedimentation Sodium Bottle Posaconazole % Label Benzoate % Label Number Time mg/ml Strength mg/ml Strength 1 0 min 39.9 99.8 2.00 100 1 5 min 40.0 100 1.99 99.5 1 10 min 40.0 100 2.00 100 1 30 min 40.0 100 2.00 100 1 60 min 40.2 101 2.01 101 1 3 days 40.2 101 2.02 101 2 0 min 39.8 99.5 2.01 101 2 5 min 39.9 99.8 2.00 100 2 10 min 40.2 101 2.01 101 2 30 min 39.8 99.5 1.99 99.5 2 60 min 40.2 101 2.02 101 2 3 days 40.1 100 2.01 101 - Two bottles containing the suspension of the present invention were shaken and left to rest. The bottles were then sampled immediately (time zero), then after 5 minutes, 10 minutes, 30 minutes, 60 minutes and after 72 hours (three days) post shaking. The levels of posaconazole and of the preservative (Sodium Benzoate) in these samples were assayed by HPLC. HPLC methods of detection are well-known to one of skill in the art.
- The results of the assay of the preservative and of the posaconazole remained consistent and did not change. These ranged from 39.8 to 40.2 mg/ml (99.5 to 101%) for the active and 1.99 to 2.02 mg/m: (99.5 to 101%) for the preservative, respectively. The results of this experiment are shown in Table 1 above.
- The sodium benzoate was not expected to sediment. Surprisingly, the posaconazole was not sedimented after 3 days.
- Thus, the compositions of the present invention have both ease of dispersibility and homogeneity as is evidenced by the stability of the samples in Table 1.
- Next, accelerated homogeneity testing was performed on the liquid suspension of the present invention.
TABLE 2 Posaconazole Oral Suspension 40 mg/ml Homogeneity Accelerated Posaconazole Sodium Benzoate Condition Time point % Label Strength % Label Strength Initial Initial 103; 102; 104 105; 102; 103 30 H (30° C./60% 3 Months 103; 105; 104 103; 107; 105 RH) RH4 (40° C./75% 3 Months 102; 104; 103 104; 106; 106 RH) 30 H 6 Months 102; 101; 102 103; 101; 102 RH4 6 Months 102; 102; 102 101; 101; 102 25 H 12 Months 104; 104; 104 101; 100; 100 25 H 24 Months 104; 104; 104 101; 101; 101 - These data (sedimentation rate experiment) were in agreement with the real time stability data (up to 6 months at 40° C./75% RH and up to 24 months at 25° C./60% RH) that are shown in Table 2. The assay homogeneity results, surprisingly, remained consistently homogenous and practically unchanged.
- After 6 months at 40° C./75% RH the homogeneity results ranged from 40.7 to 40.8 mg/ml (101%) for the active and 2.01 to 2.03 mg/ml (101 to 102%) for preservative, respectively. These results were obtained regardless of the portion of the bottle assayed, i.e., top, or bottom of the bottle. Therefore, it can be concluded that the suspension was homogenous throughout the bottle even after relatively long exposure to accelerated stability conditions.
- After 24 months at 25° C./60% RH the homogeneity results ranged from 41.5 to 41.6 mg/ml (104%) for the active and 2.01 mg/ml (101%) for the preservative, respectively. These results were obtained regardless of the portion of the bottle assayed, i.e., top or bottom of the bottle. Therefore, it can be concluded that the suspension was homogenous throughout the bottle even after long term (24 months) exposure to 25° C./60% RH.
- Bioavailability is defined as the rate and extent to that the active drug ingredient or therapeutic moiety is absorbed into the systemic circulation from an administered dosage form as compared to a standard or control.
- C max value is defined as the maximum concentration of the antifungal compound measured (i.e. “peak”) in the plasma serum.
- Formulations of the present invention have the advantage that they have an increased bioavailability and lower variability than previous formulations.
- The relative bioavailability of the posaconazole oral suspension was compared to a solid dosage form of posaconazole in healthy subjects.
- The first objective was to determine the relative bioavailability of posaconazole given as an oral suspension compared to an oral solid formulation when administered with a high-fat breakfast. The second objective was to determine the effect of high-fat and non-fat food relative to fasting conditions on the oral bioavailability of the compound of formula I when given as an oral suspension.
- Twenty healthy subjects completed this randomized, open-label, 4-way crossover, single-dose bioavailability and food effect study of posaconazole. Subjects received each of the following four treatments separated by at least a 7 day washout period:
Treatment A: 200 mg of the compound of formula I in the oral suspension of this invention (5 ml) following a 10-hr. fast Treatment B: 200 mg of the compound of formula I in the oral suspension of this invention (5 ml) with a standardized high-fat breakfast Treatment C: 200 mg of the compound of formula I in the oral suspension of this invention (5 ml) with a standardized non-fat breakfast Treatment D: 2 × 100 mg of the compound of formula I in the tablets (co-precipitate formulation of U.S. Pat. No. 5,834,472 with a standardized high-fat breakfast - Subjects were randomized to either remain fasted (Treatment A), to receive a standardized high fat breakfast (Treatment B or D) or a standardized non-fat breakfast (Treatment C). Meals were consumed in a 20-minute period prior to the morning drug administration and subjects received the appropriate treatment within 5 minute of completing the breakfast.
- Blood samples (6 ml) were collected into heparinized tubes for each treatment immediately prior to dosing (0 hour) and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 24, 36, 48 and 72 hours after dosing. Blood was centrifuged at 4° C. and plasma stored at or below −20° C. until assayed. Plasma concentrations of posaconazole were assayed using a validated high performance liquid chromatographic assay with a LOQ of ng/ml.
- Individual plasma concentration-time data were used for pharmacokinetic analysis using model-independent methods. The maximum concentration (Cmax) and time of maximum concentration (Tmax) were the observed values. The area under the plasma concentration-time curve from time zero to the final quantifiable sampling time [AUC(tf)] was calculated using the linear trapezoidal method and extrapolated to infinity (I) as follows:
- where C(tf) is the estimated concentration determined from linear regression at time, tf.
- Total body clearance was calculated by the following:
- CL=Dose/AUC(I)
- The apparent volume of distribution (Vdarea/F) was calculated from the ratio of the total body clearance to the terminal phase rate constant (K).
- Summary statistics were calculated for the plasma suspension formulation of the present invention compared to a prior art tablet formulation concentration-time data at each time point and the derived pharmacokinetic parameters. The original scale and log-transformed Cmax and AUC values were analyzed using an analysis of variance (ANOVA). The effects due to subject, phase and treatment were extracted.
- The plasma concentration-time data and pharmacokinetic parameters for the compound of formula I are tabulated in Tables 3 & 4 and depicted graphically in FIGS. 1 & 2.
- All subjects had 0-hour concentrations on Day 1 reported as below the LOQ (5 ng/ml) except for Subject 20 in Phases 3 and 4 who had quantifiable levels of posaconazole at 0-hour for Treatments B and A of 8.5 and 22.5 ng/ml, respectively. These levels are most likely due to a carry-over effect from accumulation from previous doses.
- A summary of the mean pharmacokinetic parameters are provided in the table below:
TABLE 3 Tablet Suspension High Fat D 10 hr Fast A High Fat B Non-Fat C Parameter Unit Mean % CV Mean % CV Mean % CV Mean % CV Cmax ng/ml 413 33 132 50 512 34 378 43 Tmax Hr 5.5 32 5.01 49 4.8 9 4.1 21 AUC(tf) ng-hr/ml 10304 41 3553 36 13885 41 9511 38 AUC(l) ng-hr/ml 11832 39 4179 31 15059 26 10753 35 t½ hr 21.0 15 23.5 25 23.0 19 22.2 18 - Posaconazole was slowly absorbed; the mean Tmax values ranged from 4.1 to 5.5 hr. Posaconazole was slowly eliminated with a mean terminal t½ of about 22 hour which was independent of treatment. This study was conducted to evaluate the bioavailability of posaconazole oral suspension (Treatment B) compared to a tablet formulation (Treatment D), both given with a high-fat food. The results, based on log-transformed data, are shown below:
TABLE 4 Treatments Given After Relative 90% High Fat Geometric Bioavailability Confidence Parameter Breakfast Mean (%)b Interval Cmax Suspension 485 ng/ml 123.3 104-146 Tablet 394 ng/ml AUC(tf)a Suspension 13141 ng.hr/ml 136.5 119-156 Tablet 9624 ng.hr/ml - On average, the suspension formulation of the present invention resulted in a 23% increase in Cmax and a 36% increase in AUC(tf) compared to the tablet of the prior art.
- The secondary objective of the study was to evaluate the effect of high fat food (Treatment B) and non-fat food (Treatment C) compared to fasting (Treatment A) on the oral bioavailability of posaconazole administered as an oral suspension. The results, based on log-transformed data, are shown below:
TABLE 5 Relative 90% Suspension Geometric Bioavailability Confidence Parameter Treatments Mean (%)a Interval Cmax High-Fat 485 417 352-493 (ng/ml) Non-Fat 345 296 250-350 Fast 116 — — AUC(tf) High-Fat 13141 392 343-448 (ng.hr/ml Non-Fat 8857 264 231-302 Fast 3352 — — - A high fat breakfast produced a 4-fold increase in the bioavailability of posaconazole given in a suspension. This was consistent with results from a previous study where food significantly increased the bioavailability of posaconazole by 3-5-fold for both tablet and capsule formulations. The effect of a non-fat breakfast (Treatment C) compared to fasting (Treatment A) was less, with a 2.5-3-fold increase in bioavailability.
- Many modifications and variations of this invention can be made without departing from its spirit and scope, as will be apparent to one skilled in the art. The specific embodiments described herein are offered by way of example only, and the invention is to be limited only by the terms of the appended claims along with the full scope of equivalents to which such claims are entitled.
Claims (20)
2. The liquid suspension of claim 1 , wherein the at least one non-ionic surfactant is a block copolymer of ethylene oxide and propylene oxide, glycol or glyceryl esters of saturated or unsaturated C8to C20 acids, polyoxyethylene esters of saturated or unsaturated C8to C20 acids, polyoxyethylene ethers of saturated or unsaturated C8to C20 acids, polyvinylalcohols or sorbitan esters of saturated or unsaturated C10 to C20 acids.
3. The liquid suspension of claim 1 , wherein the at least one non-ionic surfactant is a polyoxyethylene derivative of a sorbitan ester of a saturated or unsaturated C10to C20 acid.
4. The liquid suspension of claim 1 , wherein the at least one thickening agent is selected from xanthan gum, liquid sugars, starches, celluloses and mixtures thereof.
5. The liquid suspension of claim 1 , wherein a combination of xanthan gum and a liquid sugar are used as the at least one thickening agent.
6. The liquid suspension of claim 1 , wherein the micronized compound of formula I has a mean particle size of about 1200 nm to about 1600 nm.
7. A liquid suspension comprising:
(a) an antifungally effective amount of the micronized compound represented by the chemical structural formula I:
(b) an effective amount of at least one thickening agent;
(c) an amount of a buffer system effective to maintain the pH of the system in the range of about 4.0 to about 6.0;
(d) an effective amount of at least one non-ionic surfactant; and
(e) a pharmaceutically acceptable liquid carrier.
8. The liquid suspension of claim 7 , wherein the at least one non-ionic surfactant is a polyoxyethylene derivative of a sorbitan ester of a saturated or unsaturated C10to C20 acid.
9. The liquid suspension of claim 7 , wherein the sorbitan ester is a fatty acid ester of sorbitan selected from sorbitan monolaurate, sorbitan monooleate, sorbitan sesquioleate, sorbitan trioleate, sorbitan monopalmitate, sorbitan monostearate and sorbitan tristearate, or mixtures thereof.
10. The liquid suspension of claim 7 , wherein the at least one thickening agent is selected from gums, liquid sugars, starches, cellulose and mixtures thereof.
11. The liquid suspension of claim 7 , wherein a combination of a xanthan gum and a liquid sugar is used as the at least one thickening agent.
12. The liquid suspension of claim 7 , wherein a combination of a xanthan gum and a liquid glucose is used as the thickening agent.
13. The liquid suspension of claim 7 , wherein the buffer system comprises sodium citrate and citric acid.
14. The liquid suspension of claim 7 , wherein the pharmaceutically acceptable liquid carrier is a combination of purified water, glucose and glycerin.
15. The liquid suspension of claim 7 , wherein the micronized compound of formula I has a mean particle size of about 1200 nm to about 1600 nm.
16. A liquid suspension comprising:
(a) an antifungally effective amount of the micronized compound comprising chemical structural formula I:
wherein the micronized compound of formula I has a mean particle size in the range of about 1200 nm to about 1600 nm,
(b) an effective amount of a polyoxyethylene derivative of sorbitan esters of saturated or unsaturated C12 to C18 acids;
(c) an effective amount of a buffer system sufficient to maintain a pH in the range of about 4.0 to about 6.0;
(d) an effective amount of a combination of two thickening agents, wherein one is a liquid sugar; and
(e) a pharmaceutically acceptable liquid carrier.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/114,612 US20030055067A1 (en) | 2001-04-03 | 2002-04-01 | Antifungal composition with enhanced bioavailability |
| US13/161,097 US8263600B2 (en) | 2001-04-03 | 2011-06-15 | Antifungal composition with enhanced bioavailability |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US28113901P | 2001-04-03 | 2001-04-03 | |
| US10/114,612 US20030055067A1 (en) | 2001-04-03 | 2002-04-01 | Antifungal composition with enhanced bioavailability |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/161,097 Continuation US8263600B2 (en) | 2001-04-03 | 2011-06-15 | Antifungal composition with enhanced bioavailability |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20030055067A1 true US20030055067A1 (en) | 2003-03-20 |
Family
ID=23076102
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/114,612 Abandoned US20030055067A1 (en) | 2001-04-03 | 2002-04-01 | Antifungal composition with enhanced bioavailability |
| US13/161,097 Expired - Lifetime US8263600B2 (en) | 2001-04-03 | 2011-06-15 | Antifungal composition with enhanced bioavailability |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/161,097 Expired - Lifetime US8263600B2 (en) | 2001-04-03 | 2011-06-15 | Antifungal composition with enhanced bioavailability |
Country Status (18)
| Country | Link |
|---|---|
| US (2) | US20030055067A1 (en) |
| EP (2) | EP2090165A3 (en) |
| JP (2) | JP4308902B2 (en) |
| KR (1) | KR100607742B1 (en) |
| CN (1) | CN100415234C (en) |
| AR (1) | AR033096A1 (en) |
| AU (1) | AU2002257104B2 (en) |
| BR (1) | BR0208626A (en) |
| CA (1) | CA2443089C (en) |
| HU (1) | HUP0400856A3 (en) |
| MX (1) | MXPA03009021A (en) |
| NO (1) | NO20034419L (en) |
| NZ (1) | NZ528363A (en) |
| PE (1) | PE20020994A1 (en) |
| PL (1) | PL212985B1 (en) |
| TW (1) | TWI311464B (en) |
| WO (1) | WO2002080678A1 (en) |
| ZA (1) | ZA200307684B (en) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005117831A1 (en) * | 2004-05-28 | 2005-12-15 | Schering Corporation | Injectable pharmaceutical suspension comprising posaconazole |
| US20060160823A1 (en) * | 2004-05-28 | 2006-07-20 | Leonore Witchey-Lakshmanan | Particulate-stabilized injectable pharmaceutical compositions of Posaconazole |
| US20070065507A1 (en) * | 2005-09-16 | 2007-03-22 | Selamine Limited | Bisphosphonate formulation |
| US20070281011A1 (en) * | 2006-05-30 | 2007-12-06 | Elan Pharma International Ltd. | Nanoparticulate posaconazole formulations |
| US20080167364A1 (en) * | 2006-12-01 | 2008-07-10 | Selamine Limited | Ramipril-amine salts |
| US20080171775A1 (en) * | 2006-12-01 | 2008-07-17 | Selamine Limited | Ramipril-amlodipine salt |
| US20080188539A1 (en) * | 2006-12-01 | 2008-08-07 | Selamine Limited | Ramipril-amino acid salts |
| WO2009129300A2 (en) * | 2008-04-15 | 2009-10-22 | Schering Corporation | High density compositions containing posaconazole and formulations comprising the same |
| US20110034478A1 (en) * | 2008-04-15 | 2011-02-10 | Schering-Plough Corporation | Oral Pharmaceutical Compositions in a Solid Dispersion Comprising Preferably Posaconazole and HPMCAs |
Families Citing this family (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9700866B2 (en) | 2000-12-22 | 2017-07-11 | Baxter International Inc. | Surfactant systems for delivery of organic compounds |
| US20030072807A1 (en) * | 2000-12-22 | 2003-04-17 | Wong Joseph Chung-Tak | Solid particulate antifungal compositions for pharmaceutical use |
| US8067032B2 (en) | 2000-12-22 | 2011-11-29 | Baxter International Inc. | Method for preparing submicron particles of antineoplastic agents |
| IL160570A0 (en) | 2001-09-26 | 2004-07-25 | Baxter Int | Preparation of submicron sized nanoparticles via dispersion and solvent or liquid phase removal |
| US20060009469A1 (en) * | 2004-05-28 | 2006-01-12 | Leonore Witchey-Lakshmanan | Particulate-stabilized injectable pharmacutical compositions of posaconazole |
| KR101403135B1 (en) | 2006-06-14 | 2014-06-19 | 인터벳 인터내셔널 비.브이. | A suspension comprising benzimidazole carbamate and a polysorbate |
| FR2910323A1 (en) * | 2006-12-26 | 2008-06-27 | Virbac Sa Sa | Liquid anhydrous composition, useful e.g. to prepare oral medications that are diluted with drinking water to prevent/treat diseases of livestock, comprises active ingredient dissolved in organic solvents, surfactant and xanthan gum |
| DE102008004893A1 (en) | 2008-01-17 | 2009-07-23 | Add Technologies Ltd. | Carrier pellets, process for their preparation and their use |
| EP2130540A1 (en) | 2008-06-02 | 2009-12-09 | Sandoz AG | Pharmaceutical compositions containing a crystalline form of posaconazole |
| EP2141159A1 (en) | 2008-07-03 | 2010-01-06 | Sandoz AG | A Crystalline form of posaconazole |
| US20120101277A1 (en) | 2009-07-09 | 2012-04-26 | Sandoz Ag | Crystalline form of posaconazole |
| CN102892750B (en) | 2010-05-19 | 2016-03-02 | 桑多斯股份公司 | Prepare the method for chirality hydrazides |
| RU2585683C2 (en) | 2010-05-19 | 2016-06-10 | Сандоз Аг | Cleaning of posaconazole and intermediate products for synthesis of posaconazole |
| CA2798007C (en) | 2010-05-19 | 2018-10-23 | Sandoz Ag | Preparation of posaconazole intermediates |
| CN102906087B (en) | 2010-05-19 | 2016-03-23 | 桑多斯股份公司 | Prepare the method for chiral triazole ketone |
| CN103635465A (en) | 2011-06-16 | 2014-03-12 | 桑多斯股份公司 | Process for the preparation of a chiral compound |
| EP2861213A1 (en) | 2012-06-14 | 2015-04-22 | Sandoz AG | Pharmaceutical composition comprising crystalline posaconazole |
| HUE048905T2 (en) | 2014-12-05 | 2020-08-28 | Pulmocide Ltd | Antimycotic compound |
| MX2017014814A (en) | 2015-05-21 | 2018-02-15 | Pulmocide Ltd | Antifungal 4-(4-(4-(((3r,5r)-5-((1 h -1,2,4-triazol-1 -yl)methyl)-5-(2,4-difluorophenyl)tetrahydrofuran-3-yl)methoxy)- 3-methylphenyl)piperazin-1 -yl )-n -(2-h yd roxycyc lo h exyl) b e nzam i d e, or a pharmaceutically acceptable salt thereof. |
| CN105030668B (en) * | 2015-06-26 | 2018-03-27 | 济川药业集团有限公司 | Oral posaconazole supensoid agent and preparation method thereof |
| CN106333925B (en) * | 2015-07-10 | 2019-06-18 | 上海美悦生物科技发展有限公司 | A kind of posaconazole liquid suspension and preparation method thereof |
| MX2018008105A (en) | 2015-12-30 | 2018-11-12 | Vps 3 Inc | Metalloenzyme inhibitor compounds. |
| CN106389390A (en) * | 2016-06-30 | 2017-02-15 | 重庆华邦制药有限公司 | Posaconazole coating agent and preparation method thereof |
| CN107625729A (en) * | 2017-09-29 | 2018-01-26 | 重庆华邦制药有限公司 | Oral posaconazole suspension and preparation method thereof |
| CN110507609B (en) * | 2018-05-21 | 2021-09-17 | 上海医药工业研究院 | Preparation method of posaconazole oral suspension |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4788220A (en) * | 1987-07-08 | 1988-11-29 | American Home Products Corporation (Del.) | Pediatric ibuprofen compositions |
| US5145684A (en) * | 1991-01-25 | 1992-09-08 | Sterling Drug Inc. | Surface modified drug nanoparticles |
| US5374659A (en) * | 1989-06-28 | 1994-12-20 | Mcneil-Ppc, Inc. | Aqueous pharmaceutical suspension for substantially water insoluble pharmaceutical actives |
| US5580579A (en) * | 1995-02-15 | 1996-12-03 | Nano Systems L.L.C. | Site-specific adhesion within the GI tract using nanoparticles stabilized by high molecular weight, linear poly (ethylene oxide) polymers |
| US5645856A (en) * | 1994-03-16 | 1997-07-08 | R. P. Scherer Corporation | Delivery systems for hydrophobic drugs |
| US5834472A (en) * | 1996-05-24 | 1998-11-10 | Schering Corporation | Antifungal composition with enhanced bioavailability |
| US5922709A (en) * | 1995-11-20 | 1999-07-13 | Banyu Pharmaceutical Co., Ltd. | Antifungal composition |
| US20010036966A1 (en) * | 1997-05-14 | 2001-11-01 | Yasueda Shin-Ichi | Aqueous suspension with good redispersibility |
| US20050048126A1 (en) * | 2000-12-22 | 2005-03-03 | Barrett Rabinow | Formulation to render an antimicrobial drug potent against organisms normally considered to be resistant to the drug |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4533254A (en) | 1981-04-17 | 1985-08-06 | Biotechnology Development Corporation | Apparatus for forming emulsions |
| US5552160A (en) | 1991-01-25 | 1996-09-03 | Nanosystems L.L.C. | Surface modified NSAID nanoparticles |
| AU642066B2 (en) | 1991-01-25 | 1993-10-07 | Nanosystems L.L.C. | X-ray contrast compositions useful in medical imaging |
| US5399363A (en) | 1991-01-25 | 1995-03-21 | Eastman Kodak Company | Surface modified anticancer nanoparticles |
| WO1995017407A1 (en) * | 1993-12-21 | 1995-06-29 | Schering Corporation | Tetrahydrofuran antifungals |
| US5661151A (en) | 1993-12-21 | 1997-08-26 | Schering Corporation | Tetrahydrofuran antifungals |
| US5846971A (en) | 1996-06-28 | 1998-12-08 | Schering Corporation | Oral antifungal composition |
| CA2333648C (en) | 1998-05-29 | 2008-10-21 | Rtp Pharma Inc. | Thermoprotected microparticle compositions and process for terminal steam sterilization thereof |
-
2002
- 2002-04-01 NZ NZ528363A patent/NZ528363A/en not_active IP Right Cessation
- 2002-04-01 CA CA2443089A patent/CA2443089C/en not_active Expired - Lifetime
- 2002-04-01 AU AU2002257104A patent/AU2002257104B2/en not_active Expired
- 2002-04-01 MX MXPA03009021A patent/MXPA03009021A/en active IP Right Grant
- 2002-04-01 KR KR1020037012936A patent/KR100607742B1/en active IP Right Grant
- 2002-04-01 BR BR0208626-3A patent/BR0208626A/en not_active Application Discontinuation
- 2002-04-01 CN CNB028077407A patent/CN100415234C/en not_active Expired - Lifetime
- 2002-04-01 EP EP09158184A patent/EP2090165A3/en not_active Withdrawn
- 2002-04-01 HU HU0400856A patent/HUP0400856A3/en not_active Application Discontinuation
- 2002-04-01 JP JP2002578726A patent/JP4308902B2/en not_active Expired - Lifetime
- 2002-04-01 US US10/114,612 patent/US20030055067A1/en not_active Abandoned
- 2002-04-01 WO PCT/US2002/010093 patent/WO2002080678A1/en active IP Right Grant
- 2002-04-01 PL PL363965A patent/PL212985B1/en unknown
- 2002-04-01 EP EP02726689A patent/EP1372394A1/en not_active Ceased
- 2002-04-02 TW TW091106644A patent/TWI311464B/en not_active IP Right Cessation
- 2002-04-02 PE PE2002000263A patent/PE20020994A1/en active IP Right Grant
- 2002-04-02 AR ARP020101198A patent/AR033096A1/en not_active Application Discontinuation
-
2003
- 2003-10-01 ZA ZA2003/07684A patent/ZA200307684B/en unknown
- 2003-10-02 NO NO20034419A patent/NO20034419L/en not_active Application Discontinuation
-
2008
- 2008-02-18 JP JP2008036719A patent/JP2008120836A/en active Pending
-
2011
- 2011-06-15 US US13/161,097 patent/US8263600B2/en not_active Expired - Lifetime
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4788220A (en) * | 1987-07-08 | 1988-11-29 | American Home Products Corporation (Del.) | Pediatric ibuprofen compositions |
| US5374659A (en) * | 1989-06-28 | 1994-12-20 | Mcneil-Ppc, Inc. | Aqueous pharmaceutical suspension for substantially water insoluble pharmaceutical actives |
| US5145684A (en) * | 1991-01-25 | 1992-09-08 | Sterling Drug Inc. | Surface modified drug nanoparticles |
| US5645856A (en) * | 1994-03-16 | 1997-07-08 | R. P. Scherer Corporation | Delivery systems for hydrophobic drugs |
| US5580579A (en) * | 1995-02-15 | 1996-12-03 | Nano Systems L.L.C. | Site-specific adhesion within the GI tract using nanoparticles stabilized by high molecular weight, linear poly (ethylene oxide) polymers |
| US5922709A (en) * | 1995-11-20 | 1999-07-13 | Banyu Pharmaceutical Co., Ltd. | Antifungal composition |
| US5834472A (en) * | 1996-05-24 | 1998-11-10 | Schering Corporation | Antifungal composition with enhanced bioavailability |
| US20010036966A1 (en) * | 1997-05-14 | 2001-11-01 | Yasueda Shin-Ichi | Aqueous suspension with good redispersibility |
| US20050048126A1 (en) * | 2000-12-22 | 2005-03-03 | Barrett Rabinow | Formulation to render an antimicrobial drug potent against organisms normally considered to be resistant to the drug |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060160823A1 (en) * | 2004-05-28 | 2006-07-20 | Leonore Witchey-Lakshmanan | Particulate-stabilized injectable pharmaceutical compositions of Posaconazole |
| WO2005117831A1 (en) * | 2004-05-28 | 2005-12-15 | Schering Corporation | Injectable pharmaceutical suspension comprising posaconazole |
| US20090053162A1 (en) * | 2005-09-16 | 2009-02-26 | Selamine Limited | Bisphosphonate Formulation |
| US20070065507A1 (en) * | 2005-09-16 | 2007-03-22 | Selamine Limited | Bisphosphonate formulation |
| US8093228B2 (en) | 2005-09-16 | 2012-01-10 | Selamine Limited | Bisphosphonate formulation |
| US7473684B2 (en) * | 2005-09-16 | 2009-01-06 | Selamine Limited | Bisphosphonate formulation |
| US20070281011A1 (en) * | 2006-05-30 | 2007-12-06 | Elan Pharma International Ltd. | Nanoparticulate posaconazole formulations |
| EP2343053A1 (en) | 2006-05-30 | 2011-07-13 | Elan Pharma International Limited | Nanoparticulate posaconazole formulations |
| US20080188539A1 (en) * | 2006-12-01 | 2008-08-07 | Selamine Limited | Ramipril-amino acid salts |
| US20080171775A1 (en) * | 2006-12-01 | 2008-07-17 | Selamine Limited | Ramipril-amlodipine salt |
| US20080167364A1 (en) * | 2006-12-01 | 2008-07-10 | Selamine Limited | Ramipril-amine salts |
| WO2009129300A2 (en) * | 2008-04-15 | 2009-10-22 | Schering Corporation | High density compositions containing posaconazole and formulations comprising the same |
| WO2009129300A3 (en) * | 2008-04-15 | 2010-02-11 | Schering Corporation | Oral pharmaceutical compositions in a solid dispersion comprising preferably posaconazole and hpmcas |
| US20110034478A1 (en) * | 2008-04-15 | 2011-02-10 | Schering-Plough Corporation | Oral Pharmaceutical Compositions in a Solid Dispersion Comprising Preferably Posaconazole and HPMCAs |
| US20110123627A1 (en) * | 2008-04-15 | 2011-05-26 | Larry Yun Fang | High density compositions containing posaconazole and formulations comprising the same |
Also Published As
| Publication number | Publication date |
|---|---|
| NZ528363A (en) | 2005-04-29 |
| TWI311464B (en) | 2009-07-01 |
| PL212985B1 (en) | 2012-12-31 |
| NO20034419D0 (en) | 2003-10-02 |
| CA2443089C (en) | 2011-11-01 |
| US8263600B2 (en) | 2012-09-11 |
| PL363965A1 (en) | 2004-11-29 |
| KR100607742B1 (en) | 2006-08-01 |
| JP2004527525A (en) | 2004-09-09 |
| EP2090165A2 (en) | 2009-08-19 |
| JP2008120836A (en) | 2008-05-29 |
| US20110244004A1 (en) | 2011-10-06 |
| CN100415234C (en) | 2008-09-03 |
| CN1499930A (en) | 2004-05-26 |
| HUP0400856A2 (en) | 2004-08-30 |
| WO2002080678A1 (en) | 2002-10-17 |
| KR20030087052A (en) | 2003-11-12 |
| ZA200307684B (en) | 2005-03-30 |
| EP2090165A3 (en) | 2012-03-28 |
| HUP0400856A3 (en) | 2012-09-28 |
| AU2002257104B2 (en) | 2006-02-09 |
| CA2443089A1 (en) | 2002-10-17 |
| NO20034419L (en) | 2003-12-02 |
| JP4308902B2 (en) | 2009-08-05 |
| BR0208626A (en) | 2004-03-09 |
| PE20020994A1 (en) | 2002-11-01 |
| EP1372394A1 (en) | 2004-01-02 |
| AR033096A1 (en) | 2003-12-03 |
| MXPA03009021A (en) | 2004-02-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8263600B2 (en) | Antifungal composition with enhanced bioavailability | |
| AU2002257104A1 (en) | Antifungal composition with enhanced bioavailability | |
| US10238640B2 (en) | Pharmaceutical suspension composition | |
| RU2260428C2 (en) | Benzamide derivative-containing pharmaceutical composition eliciting enhanced solubility and absorption capacity in oral applying | |
| US20070099883A1 (en) | Anhydrous mometasone furoate formulation | |
| US20130252985A1 (en) | Flavoring Systems for Pharmaceutical Compositions and Methods of Making Such Compositions | |
| US20020028794A1 (en) | Megestrol acetate suspension | |
| WO2006018814A2 (en) | Oral liquid suspensions of metaxalone | |
| EP0914127B1 (en) | Antifungal composition with enhanced bioavailability | |
| HU226823B1 (en) | Combination for enhancing the bioavailability of fexofenadine and its derivatives | |
| US20100216751A1 (en) | Liquid Bevirimat Dosage Forms for Oral Administration | |
| DE60018715T2 (en) | Pharmaceutical composition containing cyclosprin as an active ingredient | |
| JP2002526423A (en) | Oxybutynin formulations and methods of use | |
| EP3644968A1 (en) | Levocloperastine fendizoate suspension having enhanced dissolution and resuspendability | |
| AU2006200354A1 (en) | Antifungal composition with enhanced bioavailability | |
| CN113509434A (en) | Nimodipine oral solution, preparation method and application thereof | |
| US11890273B2 (en) | Losartan liquid formulations and methods of use | |
| US20060141028A1 (en) | Cyclosporin formulations | |
| WO2023218482A1 (en) | Liquid oral formulation of apixaban or pharmaceutically acceptable salt thereof | |
| EP3968955A1 (en) | Pharmaceutical oral liquid solution of ivacaftor | |
| WO2023285981A1 (en) | Stable pharmaceutical liquid composition of nilotinib | |
| SK126998A3 (en) | Compositions comprising an hiv protease inhibitor such as vx 478 and a water soluble vitamin e compound such as vitamin e-tpgs |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: SCHERING CORPORATION, NEW JERSEY Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:SHARPE, STEFAN;SEQUEIRA, JOEL;HARRIS, DAVID;AND OTHERS;REEL/FRAME:015006/0344;SIGNING DATES FROM 20020321 TO 20020326 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |