-
Andrographis paniculata (Burm. F.) Nees is one of the most widely recognized medicinal plants, which has been utilized for thousands of years as a traditional Chinese medicine. It is an annual herbaceous plant belonging to the genus Andrographis, the botanical family Acanthaceae in the order of Lamiales[1]. Macroscopically, the erect herb is 50−70 cm tall. The upper part of the stem is quadrilateral, and the lower part is nearly round. The stem texture is fragile and easy to break. Leaves are simple, opposite, shortly petiolate, or almost sessile. The leaf blade is flattened or ovate-lanceolate, 2−7 cm long and 1−3 cm wide, with an acuminate apex, reticulate venation, and cuneate-decurrent base. The margin of the leaf blade is entire or undulated[1]. In the theory of traditional Chinese medicine (TCM), the medicinal property of A. paniculata belongs to 'cold', which exhibits the effect of clearing heat, detoxifying, cooling blood, and reducing swelling[1]. A. paniculata has a long history of application in TCM theory, as well as the traditional Indian medicine system-Ayurveda. A. paniculata was first introduced into the Chinese Pharmacopoeia (ChP) in 1977 and was approved by the Food and Drug Administration (FDA) to include into the United States Pharmacopoeia (USP). According to the ChP, the dried leaves and above-ground stems are made into a Chinese medicine Andrographis Herba (Chuanxinlian, CXL), which acts as a natural immune booster with anti-infection, anti-inflammatory, antiviral, and analgesic effects[2,3].
The main active constituents of CXL include lactones, diterpenoids, flavonoids, diterpenoids lactones, diterpenoid glycosides, especially labdane diterpenoids lactones such as andrographolide (C20H30O5), neoandrographolide (C26H40O8), 14-deoxyandrographolide (C20H30O4) and dehydroandrographolide (C20H28O4)[1,3]. Many studies have shown that the diterpene compound andrographolide and its derivatives are the main active components in CXL and play an essential role in treating COVID-19 infection through TCM[4]. Notably, the content of active ingredients is the core of the quality of CXL, which was proven previously to be affected by the area of growing places, the time of harvesting and processing (bud stage with the highest content of andrographolide), and the storage time. Considering that the content of andrographolide in leaves is significantly higher than that in stems, the 2015 edition of ChP requires that the proportion of leaves must be at least 30% to ensure the quality of the medicinal herb and the clinical efficacy[1]. Besides, the total amount of active andrographolide and dehydrated andrographolide in CXL must be more than 1.5%, according to the ChP. However, there is no unified international standard for CXL, and the current imperative is to establish a consistent quality standard and grade evaluation.
South India and Sri Lanka are widely acknowledged as the origin and center of genetic diversity for the plant A. paniculata, which possesses a broad range of germplasm resources. Nowadays, it is prevalent and widely spread from Southeast Asian countries (India, Sri Lanka, China, Cambodia, Malaysia, and Thailand) to North America and the West Indies[5]. Interestingly, it has been speculated that A. paniculata in Thailand and Malaysia may have come from India[6]. There are about 20 species of Andrographis plants, two of which are distributed in China, including the cultivated species (A. paniculata) and the wild types (A. laxiflora var. glomerulifera)[7]. A. paniculata was introduced into China in the 1950s and primarily cultivated in Guangxi Zhuang Autonomous Region and Guangdong Province, which produce more than 90% of China's total production (Fig. 1)[8]. Others are scattered in Fujian, Jiangxi, Sichuan, and Anhui provinces. Furthermore, the germplasm of Chinese A. paniculata is mainly from Guangdong and Hainan. After over 80 years of being introduced and cultivated, the origin of A. paniculata remains relatively singular, with a highly similar genetic background and limited potential for evolution within the population[6].
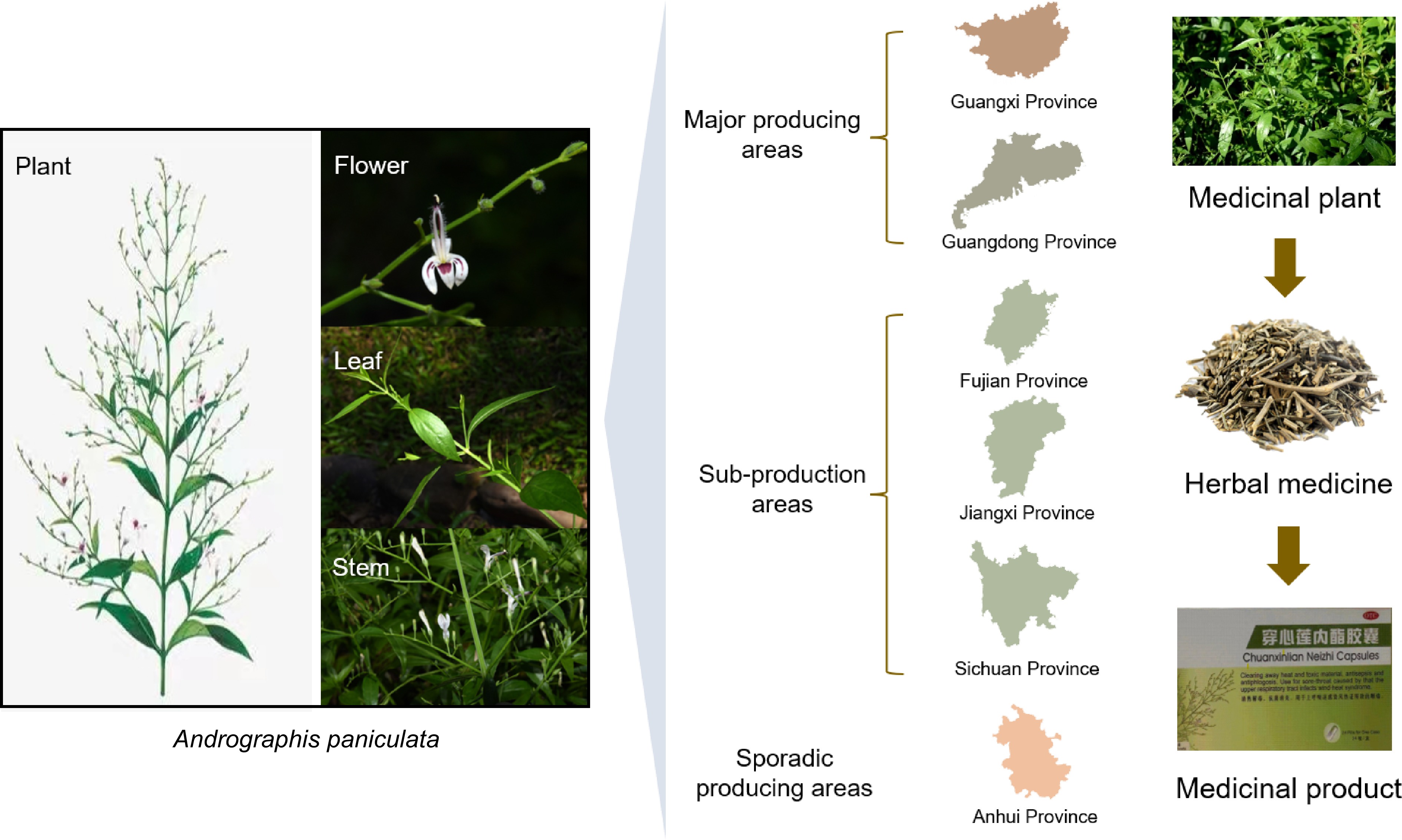
Figure 1.
Schematic diagram of the main origin in China and the industrialization process of Andrographis paniculata.
Due to the significant increase in the demand for CXL (~10,000 tons per year in China), the diversity of wild plant resources has been severely threatened in recent years. On the other hand, the long-term artificial cultivation, the different geographical regions, the natural environment, and production management have led to gene variation and isolation, interspecies hybridization, and even apparent differentiation phenomenon, resulting in unstable herb quality and heterogeneous andrographolide content of A. paniculata[5]. Therefore, collecting germplasm resources and identifying high-quality plant varieties have become the primary task of A. paniculata breeding. Germplasm materials of A. paniculata mutant have been obtained by specific mutagenesis techniques[6]. With the development of high-throughput sequencing technology, scientists have begun to study the genetic and metabolic processes of A. paniculata at the omics level. Notably, whole-genome databases have been assembled[2,9], which contained a large amount of gene annotation information and identified three terpenoid synthases, two cytochrome P450 monooxygenases, and a UDP-dependent glycosyltransferase related to andrographolide biosynthesis[2]. It is conceivable that combined with the seven basic leucine zipper (bZIP) transcription factors[10] and seven WRKY transcription factors[11] found in the transcriptomes that may be involved in regulating the andrographolide biosynthesis, more precise molecular breeding of A. paniculata and efficient heterologous synthesis of active andrographolide will be possible.
In this review, we will focus on the pharmacological activity of CXL and reveal that andrographolide and its derivatives are the main active substances of CXL, from the theory of TCM to modern pharmacological research. Subsequently, we will review the biosynthesis pathway of andrographolide and the heterologous synthesis strategies of other plant diterpenoids. It is expected that a higher yield of andrographolide can be achieved by cell factories in the future, eliminating the need to harvest A. paniculata plants.
-
Andrographis Herba (Chuanxinlian, CXL) is a herb with a nontraditional significance imported from abroad, also named Yijianxi or Sifanglian in some places[7]. The medical book Lingnan Medicinal Collection Record has recorded that CXL can cure snake bites and manage internal injury cough[12]. According to the Four Natures and Five Flavors theory of Traditional Chinese Medicine, the 'bitter' and 'cold' Chinese herbal medicine CXL belongs to the heart meridian of hand-shaoyin, the lung meridian of hand-taiyin, the large intestine meridian of hand-yangming and the bladder meridian of foot-taiyang[1]. Specifically, CXL can clear heat and detoxify, cool blood and detumescence, dry dampness, and eliminate dysentery[12]. CXL is widely used in the clinical practice of TCM, and its efficacy is like Coptidis Rhizoma (Huanglian, HL). It is usually taken orally with 6-9 g added into decoction or powder[1].
When used to clear heat and detoxify, CXL collaborates with Lonicerae Japonicae Flos (Jinyinhua) and Scutellariae Radix (Huangqin) to treat the symptoms of lung heat cough, or sore throat caused by upper respiratory tract infection, tonsillitis, bronchitis, pneumonia, and other diseases. Furthermore, as CXL plays the role of cooling blood and detumescence, it is usually used in combination with Houttuyniae Herba (Yuxingcao), Coicis Semen (Yiyiren), and Phragmitis Rhizome (Lugen) to treat lung carbuncle characterized by cough, chest pain, vomiting purulent blood and sputum. And in the function of drying dampness and eliminating dysentery, CXL always cooperates with Coptidis Rhizoma (Huanglian), Talcum (Huashi), Gardeniae Fructus (Zhizi), Artemisiae Scopariae Herba (Yinchen, YC), Phellodendri Chinensis Cortex (Huangbai) or Sophorae Flavescentis Radix (Kushen) to cure dampness-heat diarrhea, jaundice, and gonorrhea. CXL can also detoxify snake bite poisoning by external use with glycerin after grinding. In addition to these primary functions, CXL can assist other medical herbs in their therapeutic effects. For example, when decocted with Gypsum Fibrosum (Shigao), the two drugs work together to clear up excessive heat syndrome in Qi and treat the symptoms of high fever, sweating, irritability, thirst, and tachycardia. Conclusively, due to its bitter and cooling properties, CXL can guide and assist in the treatment of many damp-heat syndromes, playing a crucial role in TCM prescriptions.
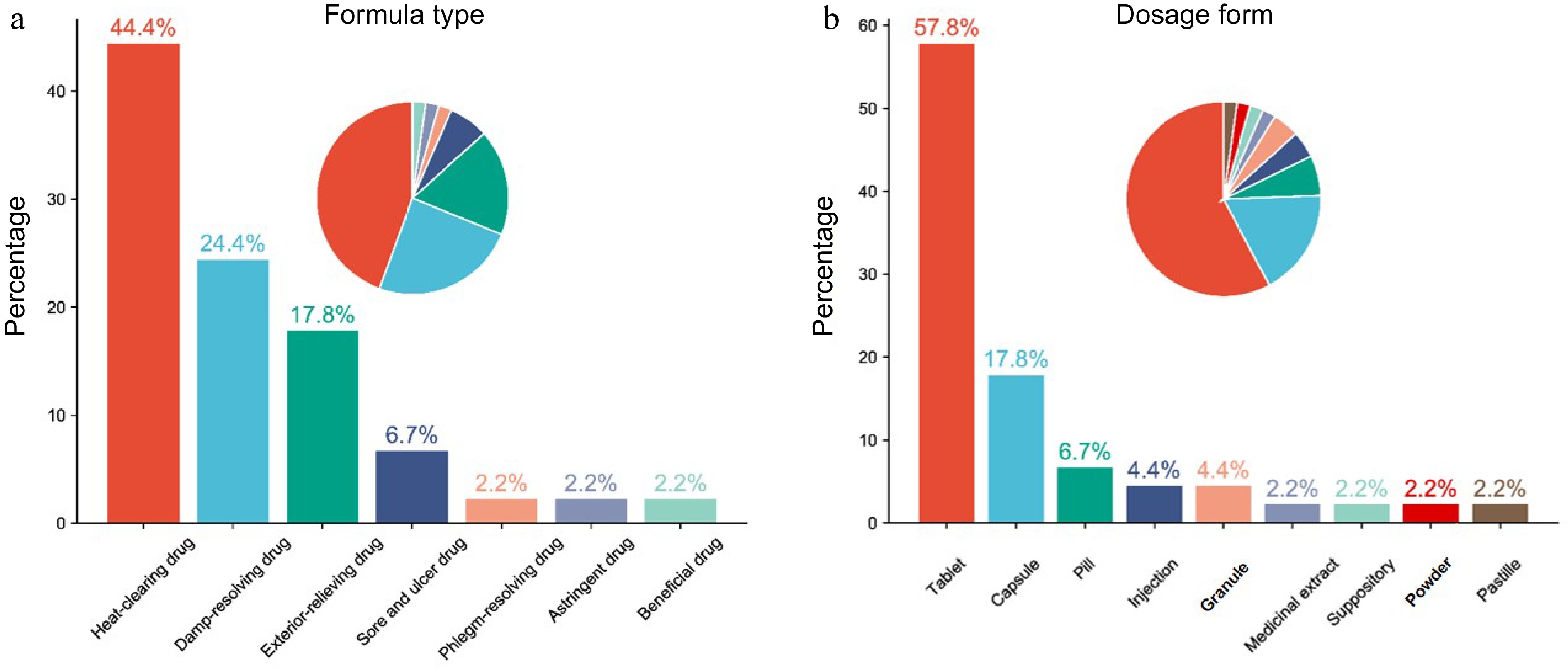
With the increasing recognition of the medicinal value of CXL, it has been used in many TCM formulas. Here, we summarized formulas containing CXL (Table 1). These formulas can be divided into seven types: heat-clearing drugs, damp-resolving drugs, exterior-relieving drugs, sore and ulcer drugs, phlegm-resolving drugs, astringent drugs, and reinforcing drugs. Meanwhile, dosage forms include tablets, capsules, pills, injections, granules, medicinal extracts, suppositories, powders, and pastilles. Among them, the most significant formula type is the heat-clearing drugs (44.4%) (Fig. 2a), while tablets dosage is the most (57.8%) (Fig. 2b). Especially one of the most famous formula tablets in China, Fufang Dantong Pian (tablet), composed of Isodi Lophanthoidis (Xihuangcao), YC, CXL, Rhei Radix et Rhizome (Dahuang) and 7-hydroxy-4-methylcoumarin (Dantong), has the effect of clearing heat, improving choleretic and relieving spastic pain. Moreover, Fufang Dantong Pian could also solve problems or diseases such as acute and chronic cholecystitis, cholangitis, gallbladder, biliary calculi complicated with infection, cholecystectomy syndrome, biliary tract dysfunction, etc[13,14]. Another typical formula containing CXL is called Ganmaoqing Pian (tablet)[15], which treats the wind-heat common cold with symptoms of fever, headache, nasal congestion, sneezing, sore throat, and body aches. Besides, there are also granules and capsules of Jinji Keli (granule)[16] and Jinji Jiaonang (capsule)[17] containing CXL, which are used to treat damp-heat adnexitis by invigorating spleen and dehumidification and activating blood circulation. Shanghai Sheyao Pian[18], a tablet with CXL as the main component, is commonly used in clinics to treat various poisonous snake bites, providing essential support for patients' health and well-being.
Table 1. TCM formulas containing Andrographis Herba (Chuanxinlian, CXL).
Formula name Components Formula type Dosage form Liaofeining Pian[19] Stemonae Radix (Baibu, BB), CXL, Imperatae Rhizoma (Baimaogen), Bletillae Rhizoma (Baiji) Reinforcing Tablet Qingre Anchuang Wan[20]
Qingre Anchuang Pian[21]Japonicae Flos (Jinyinhua, JYH), Rhei Radix et Rhizome (Dahuang, DH), CXL, Taraxaci Herba (Pugongying), Margarita (Zhenzhu, ZZ), Sophorae Tonkinensis Radix et Rhizoma (Shandougen), Glycyrrhizae Radix et Rhizoma (Gancao, GC), Gardeniae Fructus (Zhizi) Blood-activating and stasis eliminating Pill Tablet Waiyong Zijin Ding[15] Cremastrae Pseudobulbus Pleiones Pseudobulbus (Shancigu), Cinnabaris (Zhusha), Galla Chinensis (Wubeizi), Realgar (Xionghuang, XH), Knoxiae Radix (Hongdaji), CXL, Euphorbiae Semen (Qianjinzi), Notoginseng Radix et Rhizoma (Sanqi), Borneolum Syntheticum (Bingpian, BP), Caryophylli Flos (Dingxiang) Pastille Kangfu Xiaoyan Shuan[22] Sophorae Flavescentis Radix (Kushen), Patriniae Herba (Baijiangcao), Violae Herba (Zihuadiding), CXL, Taraxaci Herba (Pugongying), Suis Fellis (Zhudan), Arnebiae Radix (Zicao), ALOE (Luhui) Dampness-resolving Suppository Fufang Chuanxinlian Pian[23] CXL, Isatidis Folium (Daqingye, DQY) Tablet Fuke Qianjin Pian[24] Radix Flemingiae (Qianjinba, QJB), Zanthoxyli Radix (Danmianzhen), Rosae Laevigatae Radix (Jinyinggen, JYG), CXL, Mahoniae Caulis (Gonglaomu, GLM), Codonopsis Radix (Dangshen), Spatholobi Caulis (Jixueteng, JXT), Angelicae Sinensis Radix (Danggui) Tablet Fufang Dantong Pian[13] Isodi Lophanthoidis (Xihuangcao, XHC), Artemisia Scopariae Herba (Yinchen), CXL, DH and 7-hydroxy-4-methylcoumarin (Dantong,) Tablet Fufang Dantong Jiaonang[14] Capsule Fufang Huangqin Pian[25] Scutellariae Radix (Huangqin, HQ), Polygoni Cuspidati Rhizoma Et Radix (Huzhang), CXL, GLM Tablet Fufang Kumu Xiaoyan Pian[26] CXL, Picrasmae Ramulus et Folium (Kumu, KM) Tablet Lixieling Pian[27] Bistortae Rhizoma (Quanshen), CXL, Sophorae Flavescentis Radix (Kushen, KS) Tablet Zhilining Pian[28] CXL, KS, Aucklandiae Radix (Muxiang) Tablet Jinji Keli[16]
Jinji Jiaonang[17]JYG, JXT, QJB, GLM, Zanthoxyli Radix (Liangmianzhen), CXL Granules Capsule Xiaoyan Zhike Pian[29] Elaeagnus Pungens Folium (Hutuiziye), Platycodonis Radix (Jiegeng), Pseudostellariae Radix (Taizishen), BB, Papaveris Pericarpium (Yingsuke), Ephedrae Herba (Mahuang, MH), Fructus Viticis Negundo (Huangjingzi), Adenophorae Radix (Nanshashen), CXL Expectorant Tablet Qinggan Chuanxinlian Pian[30] CXL, Gnetum parvifolium (Maimateng) Exterior disorder-relieving Tablet Cuilian Jiedu Pian[31] Selaginellae Uncinatae Herba (Cuiyuncao), CXL, Radix Helicteris Angustifoliae (Shanzhima, SZM), Radix Et Caulis Ilicis Asprellae (Gangmei, GM), Viticis Negundo Herba (Wuzhigan), Menthae Haplocalycis Herba (Bohe, BH) Tablet Ganmaoqing Pian[32] Isatidis Radix (Banlangen, BLG), DQY, Spanishneedles Herb (Jinzhan yinpan), GM, SZM, CXL Tablet Ganmaoqing Jiaonang[32] Capsule Ganmaokang Jiaonang[33] CXL, Chrysanthemi Indici Flos (Yejuhua, YJH), Solidaginis Herba (Yizhihuanghua), Herb of Marginate Rockbell (Lanhuashen), Stauntoniae Caulis et Folium (Yemugua) Capsule Kangle Biyan Pian[34] Xanthii Fructus (Cang'erzi), Magnoliae Flos (Xinyi), Angelicae Dahuricae Radix (Baizhi), MH, CXL, HQ, Saposhnikoviae Radix (Fangfeng), Pogostemonis Herba (Guanghuoxiang), Moutan Cortex (Mudanpi), BH Heat-clearing Tablet Zhiganjia Pian[35] SZM, CXL, Tadehagi Triquetri Herba (Hulucha), Euodiae Leptae Folium et Ramulus (Sanchaku), BLG, Notopterygii Rhizoma et Radix (Qianghuo), BH Tablet Houkang San[36] BP, ZZ, Ginseng Radix et Rhizoma (Renshen), Borax (Pengsha), Natrii Sulfas Exsiccatus (Xuanmingfen), L-Menthol (Bohenao), Trichosanthis Radix (Tianhuafen), CXL, Indigo Naturalis (Qingdai), GC powder Liandan Xiaoyan Pian[37] CXL, KM Tablet Lianzhi Xiaoyan Pian[38] CXL, SZM Tablet Lianzhi Xiaoyan Jiaonang[39] Capsule Fufang Honggencao Pian[40] Alviae Prionitidis Herba (Honggencao), Houttuyniae Herba (Yuxingcao), JYH, YJH, CXL Tablet Houshuning Pian[41] Herba Hedyotidis (Baihua Sheshe cao), CXL, SZM Tablet Qianxi Pian[42] CXL, Senecionis Scandentis Hebra (Qianliguang) Tablet Qinghuo Zhimai Pian[43] CXL, ZZ, Ophiopogonis Radix (Maidong) Tablet Qinghuo Zhimai Jiaonang[44] Capsule Xiaoyan Lidan Pian[45] CXL, XHC, KM Tablet Xinxue Pian[46] Magnetitum (Cishi), Gypsum Fibrosum (Shigao), Talcum (Huashi), Gypsum Rubrum (Hanshuishi), Saltpeter (Xiaoshi), Natrii Sulfas (Mangxiao), ZZ, Lophatheri Herba (Zhuye), Radix Serratulae Chinensis (Guangshengma), CXL, ZZ, Aquilariae Lignum Resinatum (Chenxiang), BP Tablet Xinxue Keli[47] Granules Yanhou Xiaoyan Wan[48] Bufonis Venenum (Chansu), CXL, Ramulus et Folium Schefflerae (Qiyelian), ZZ, BP, XH, Plant Soot (Baicaoshuang) Pill Shang Hai She Yao Pian[18] CXL, Ecliptae Herba (Mohanlian) Tablet -
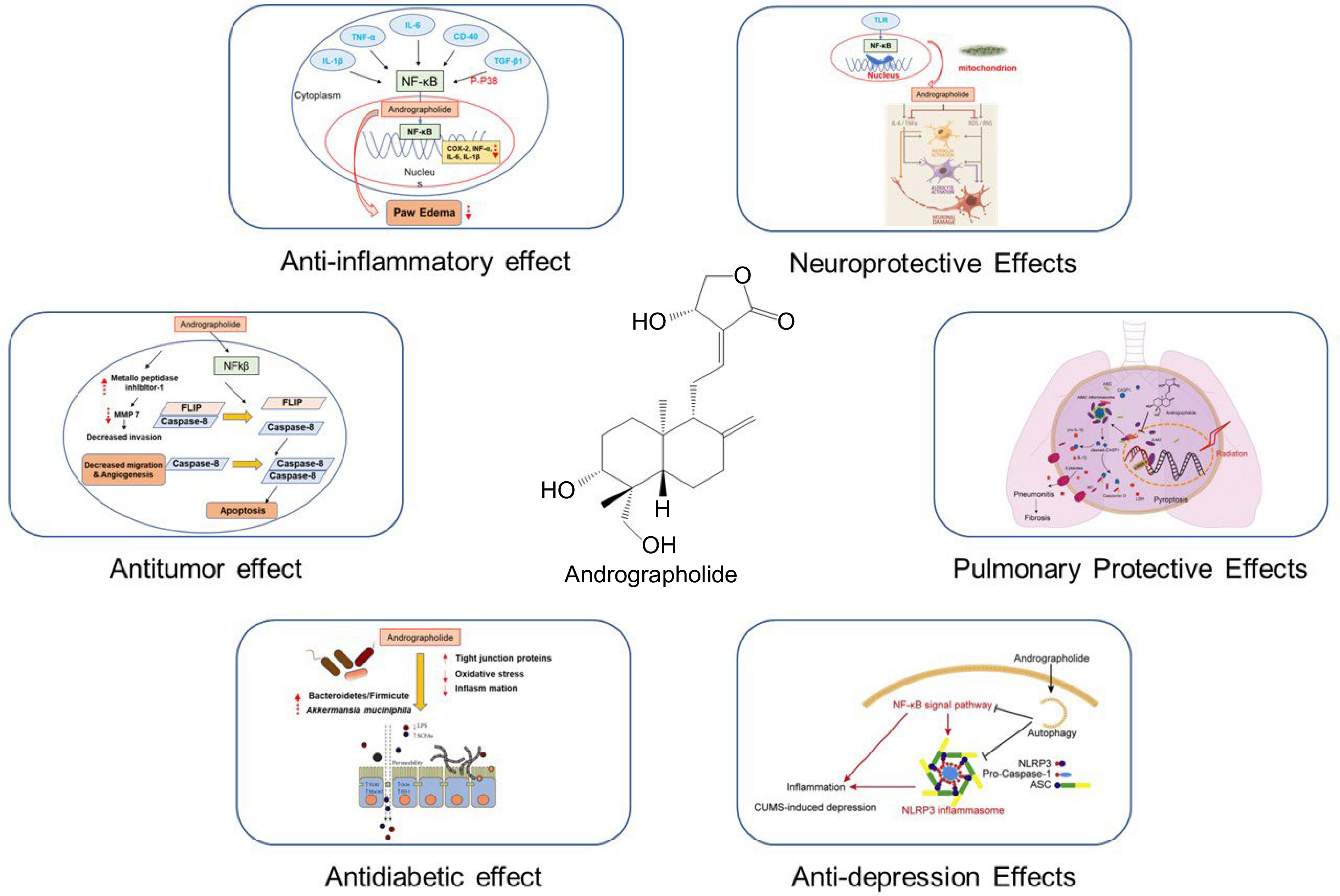
Andrographolide and its derivates are the principal bioactive compounds of the medicinal plant A. paniculata[4], which have anti-inflammatory, anti-tumor, anti-diabetes, cardiovascular protection, neuroprotective effect, hepatoprotective effect, and others (Fig. 3).
Anti-inflammatory effects and mechanism of andrographolide
-
As mediators of intercellular interactions, cytokines are crucially involved in initiating and perpetuating inflammatory disorders. The inhibition of inflammatory mediators represents an effective therapeutic strategy for both acute and chronic inflammatory conditions. Andrographolide exerts its anti-inflammatory effects through many pathways and targets, including regulating the synthesis and release of inflammatory mediators.
According to the research, andrographolide has been shown to alleviate airway inflammation by neutrophil infiltration in lung tissue in a mouse model of ovalbumin (OVA)-induced asthma by blocking cytokines regulated by helper T cell 17 (Th17) and inhibiting the expression of Janus tyrosine-protein kinase one and signal transducer and activator of transcription 3 (JAK1/STAT3) signaling pathway, thereby reducing exaggerated allergic inflammation response of the upper respiratory tract[49]. In the complete Freund's adjuvant (CFA)-induced acute paw edema inflammation model, andrographolide can significantly reduce the degree of inflammatory swelling in mice. It has exerted anti-inflammatory effects by inhibiting a series of inflammation-related molecules, such as COX-2, NF-κB, p-p38, CD40, tumor necrosis factor-α, IL-1β, and IL-6, suggesting that andrographolide possesses strong anti-inflammatory properties[50]. Inflammatory disorders are often associated with activating NOD-like receptor protein 3 (NLRP3) inflammasomes by various stimuli, including monosodium urate (MSU). Upon activation, NLRP3 inflammasomes release a large amount of interleukin (IL)-1β, contributing to the pathogenesis of numerous inflammatory diseases. In a mouse model of MSU-induced arthritis, treatment with andrographolide has been found to reduce monocyte infiltration and IL-1β release in the knee joint, indicating its potential therapeutic effect in ameliorating inflammation associated with NLRP3 inflammasome activation[51]. Moreover, the miR-27-3p/matrix metalloproteinase 13 (MMP-13) signaling axis may represent another potential therapeutic target for treating and preventing osteoarthritis progression. Andrographolide has been demonstrated to mitigate cartilage damage and stimulate chondrocyte anabolism by regulating MMP-13 through the miR-27-3p signaling pathway[52]. Additionally, andrographolide has been found to ameliorate acute colitis in mice via the activation of the adenosine monophosphate-activated protein kinase (AMPK) signaling pathway. The disease activity index (DAI) score has been significantly reduced, and colon shortening has been markedly improved in the andrographolide-treated group[53].
It can be concluded that the primary mechanism through which andrographolide exerts anti-inflammatory effects includes inhibiting inflammatory cell activity and suppressing inflammatory mediators' expression. In terms of clinical trials of drugs, the evaluation of the efficacy and safety of andrographolide sulfonate in the treatment of acute tonsillitis and acute bronchitis has entered Phase VI (NCT numbers: NCT03134443, NCT03132623). Meanwhile, the clinical study of andrographolide in treating acute exacerbations of chronic bronchitis has also entered Phase VI (NCT number: NCT03132610).
Antitumor effects and mechanism of andrographolide
-
Andrographolide has been shown to possess antitumor activity by activating, regulating, and modulating the expression of various genes. In anti-prostate cancer, andrographolide has been found to inhibit cell proliferation and migration, induce G1/G0 cell cycle arrest, and suppress tumor growth and metastasis in mice[54]. Similarly, andrographolide has been demonstrated to impede breast tumor growth and metastasis in mice and inhibit the in vitro proliferation, migration, and invasion of MCF-7 breast cancer cells[55]. Furthermore, andrographolide can block breast cancer invasion by upregulating tissue inhibitors of metalloproteinase 1 (TIMP1) and downregulating the expression of MMP-7[56]. In the case of anti-cervical cancer, andrographolide can dose-dependently inhibit cell proliferation, promote apoptosis, induce cell cycle arrest at the G1/S phase, and significantly reduce the expression of inducible nitric oxide synthase (iNOS), which is associated with poor survival and increased tumor aggressiveness in cervical cancer[57]. Besides, andrographolide has been shown to increase both early and late apoptosis of colorectal cancer cells by inducing G0/G1 phase cell cycle arrest, upregulating pro-apoptotic protein Bax and downregulating anti-apoptotic gene Bcl-2[58]. Additionally, andrographolide can work synergistically with other anti-tumor drugs to induce colon cancer cell apoptosis through nuclear condensation, phosphatidylserine externalization, and Caspase-3 activation[59]. The role of andrographolide in palliative treatment for advanced esophageal cancer has entered phase III clinical trials (NCT number: NCT04196075).
Treatment of cardiovascular diseases and diabetes with andrographolide
-
By using a cerebral ischemia-reperfusion injury (CIRI) animal model, it has been demonstrated that the protective effects of andrographolide on CIRI may be attributed to reducing neuroinflammation and cellular apoptosis[60]. Additionally, andrographolide has been found to relieve endothelial dysfunction in mice with coronary heart disease by modulating peroxisome proliferator-activated receptor (PPAR) and NF-κB signaling pathways[61]. As is well-known, inhibiting cellular apoptosis, regulating serum lipid levels, improving hemodynamics, and suppressing oxidative and inflammatory cytokine expression all play crucial roles in mitigating the pathogenesis of cardiovascular disease. Research has presented that andrographolide possesses specific abilities in regulating glucose metabolism, as demonstrated by reducing serum high-density lipoprotein cholesterol (HDL-C) levels and increasing low-density lipoprotein cholesterol (LDL-C)/HDL-C in mice[62]. Type 2 diabetes (T2DM) is closely related to intestinal barrier dysfunction. Andrographolide has been shown to improve glycemic control by enhancing intestinal barrier function and increasing microbial diversity of Akkermansia muciniphila in vitro[63]. Furthermore, nano-emulsion-based andrographolide delivery can enhance andrographolide anti-diabetic activity[64]. Notably, the evaluation of the pharmaco-metabolomics of andrographolide and metformin under fasting conditions in healthy people has entered phase 1 clinical trial evaluation (NCT number: NCT04161404).
Neuroprotective effects and mechanism of andrographolide
-
In the pathogenesis of Alzheimer's disease, andrographolide plays a role in regulating multiple vital steps. It has been shown to lower acetylcholinesterase (AChE), β-amyloid 1-42 (Aβ1-42), and p-tau levels in response to Alzheimer's disease induced by streptozotocin (STZ) in mice. This neuroprotective effect is achieved through anti-inflammatory, antioxidant, and modulation of neurotransmitter pathways[65]. Andrographolide can also significantly reduce the expression levels of total Aβ burden, IL-6, 4-hydroxynonenal, and N-tyrosine adducts in the rat brain[66]. In addition, it decreases the expression of Toll-like receptor 2 (TLR2), leukocyte differentiation antigen 14 (CD14), chemokine ligand 3 (CCL3), and Toll-like receptor 1 (TLR1) in APP/PS1 mice, thereby alleviating symptoms of Alzheimer by reducing neuroinflammation[67]. Furthermore, andrographolide exhibits an antidepressant effect by improving the mood of mice exposed to various unpredictable stressors. This antidepressant property is attributed to promoting the hippocampal brain-derived neurotrophic factor (BDNF) signaling pathway[68].
Hepatoprotective and pulmonary protective effects of andrographolide
-
Andrographolide has a beneficial effect on the model of ethanol-induced alcoholic liver disease (ALD). After drug treatment, it can improve serum aminotransferase levels, liver function, lipid accumulation, and liver reactive oxygen species levels and alleviate liver pathological damage and oxidative stress in ALD mice. The mechanism is related to the downregulation of NF-κB and TNF-α expression[69]. Multiple studies have reported the protective effects of andrographolide on lung tissue. In a rat model of pulmonary fibrosis induced by bleomycin (BLM), andrographolide can ameliorate lung tissue fibrosis by inhibiting lung fibroblast proliferation, differentiation, and extracellular matrix deposition through the regulation of Smad-dependent and Smad-independent pathways mediated by transforming growth factor-β1 (TGF-β1)[70]. Benefiting from the anti-inflammatory activity, andrographolide can significantly alleviate inflammatory lesions in lung tissue, such as pulmonary edema and alveolar wall thickening, by reducing inflammatory cell infiltration and the secretion of proinflammatory cytokines (IL-1β, IL-6)[71].
Other effects
-
Andrographolide has been suggested for use with melatonin for treating COVID-19 based on its antipyretic, anti-inflammatory, antioxidant, antiviral, and endoplasmic reticulum stress regulation properties. Docking calculations have been performed between andrographolide and the binding sites of SARS-CoV-2[72]. A phase Ⅲ clinical trial is ongoing (NCT05019326) to evaluate the effects of A. paniculate and Boesenbergia Rotunda vs control in asymptomatic COVID-19 patients. The 'Xi Yan Ping' injection is listed in the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 10) for the clinical treatment of COVID-19 in China[73]. The active ingredient of the injection is andrographolide total sulfonate, which has been proven with the functions of clearing heat, detoxification, and anti-inflammatory of patients infected with SARS-CoV-2.
Andrographolide has also shown promise in the treatment of osteoporosis by promoting bone formation and enhancing osteoblast differentiation, in which the mechanism is regulating the osteoprotegerin (OPG)/receptor activator of the NF-κB ligand (RANKL) signaling pathways[74]. In addition, andrographolide exhibits potent antimalarial activity against Plasmodium falciparum 3D7, potentially by inhibiting glycogen synthase kinase 3β (GSK3β) expression and NF-κB activity[75]. Meanwhile, it can reverse chloroquine resistance by enhancing the inhibitory effect of chloroquine on plasmodial hemoglobin formation[76].
In conclusion, andrographolide has been shown to have various pharmacological effects, including anti-inflammatory, antitumor, treatment of cardiovascular diseases and diabetes, protection of the nervous system, and hepatoprotection. However, most studies on the effects of andrographolide on the nervous and cardiovascular systems have been limited to laboratory stages using cell and animal models. Therefore, most of these pharmacological effects need more clinical research data to support them, which requires further investigation to fully understand andrographolide's absorption, distribution, and metabolic processes in vivo and the pathways through which it targets organs and exerts therapeutic effects.
-
Andrographolide is regarded as the principal constituent responsible for the therapeutic properties of CXL, which is a diterpene lactone derived from C5 unit isopentenyl diphosphate (IPP) and dimethyl allyl pyrophosphate (DMAPP). Notably, diterpenoids (C20) are a vast class of structurally diverse metabolites of medical plants and are derived from the precursor (E,E,E) ‐geranylgeranyl diphosphate (GGPP)[2]. Diterpenoids are required for plant growth or development and carry critical ecological and agronomic functions. Furthermore, diterpenoids have a wide range of commercial applications in the cosmetic, food additive, and pharmaceutical industries[77].
Like diterpenoids, the biosynthesis of andrographolide can be divided into three modules (Fig. 4a, c & d, annotated in gray color): the formation of precursor GGPP, derived from cytosolic mevalonate (MVA) pathway and plastidial 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway; the construction of carbon skeletons through cyclization and isomerization catalyzed by class II diTPSs (copalyl diphosphate synthases‐like, CPSs) and/or class I diTPSs (kaurene synthases‐like, KSLs); and the post‐modification of the molecular skeletons, including oxidation, glycosylation, and acylation. In the first module (Fig. 4a), the MVA pathway in plants starts with acetyl-CoA and is finally converted into IPP[78], which is orderly catalyzed by six enzymes including acetyl-CoA acetyltransferase (AACT), 3-hydroxy-3-methylglutaryl-CoA (HMGS), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR), mevalonate kinase (MVK), phosphomevalonate kinase (PMK), and mevalonate 5-diphosphate decarboxylase (MVD). Meanwhile, The MEP pathway consists of seven enzymatic steps orderly catalyzed by 1-deoxy-D-xylulose-5-phosphate synthase (DXS), 1-deoxy-D-xylulose-5-phosphate reductoisomerase (DXR), MEP cytidylyltransferase (MCT), 4-(cytidine 5-diphosphate)-2-C-methylerythritol kinase (CMK), 2-C-methyl-D-erythritol2,4-cyclo diphosphate synthase (MDS), hydroxymethyl butenyl 4-diphosphate synthase (HDS), and 4-hydroxy-3-methyl but-2-enyl diphosphate reductase (HDR). Subsequently, HDRs catalyze the last reaction in the MEP pathway; to be specific, HMBPP (1-hydroxy-2-methyl-2-butenyl-4-diphosphate) is converted to a mixture of IPP and DMAPP 5:1–6:1[79]. Isopentenyl diphosphate isomerase (IPI) is mainly responsible for the reversible conversion between IPP and DMAPP.
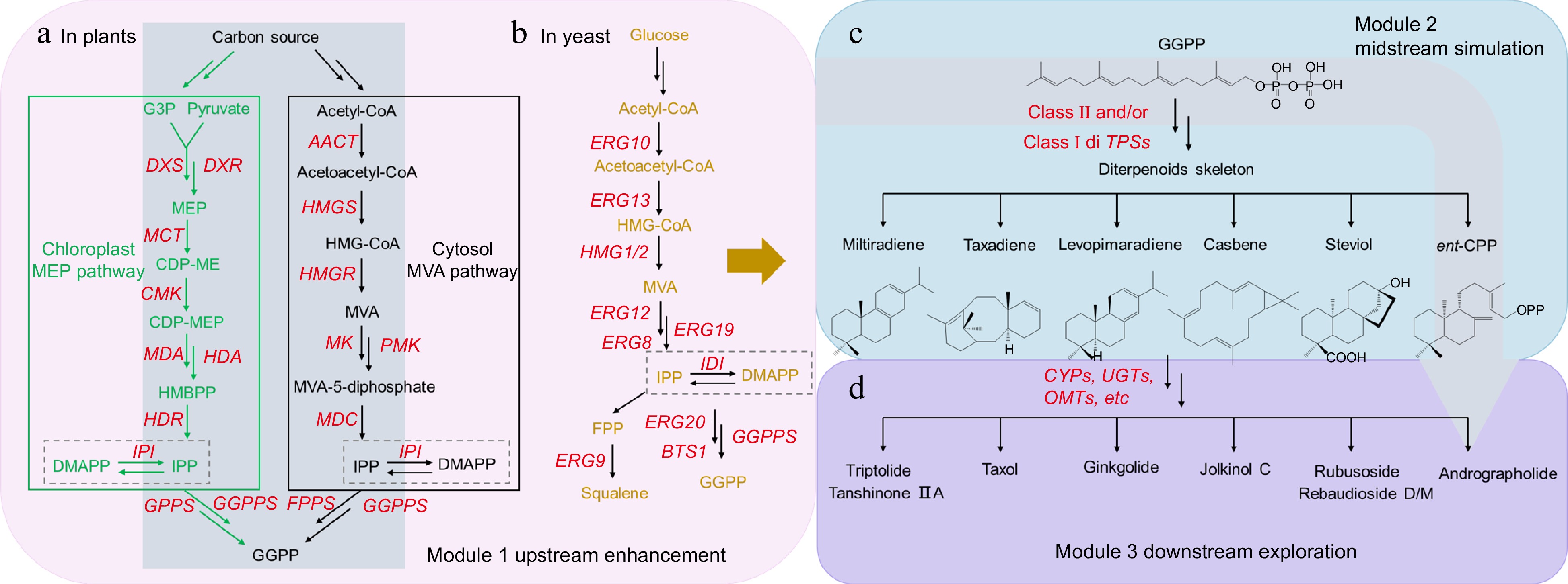
Figure 4.
Biosynthetic pathway of andrographolide (the original biosynthetic pathway of andrographolide is annotated in gray color) and heterologous synthesis strategies of diterpenoids and derivatives in microbes. (a) The formation of diterpenoid's precursor (E,E,E) -geranylgeranyl diphosphate (GGPP) in original plants. (b) The formation of GGPP in yeast. (c) The midstream module to generate diterpenoids skeletons. (d) The downstream module to create various diterpenoids with different pharmacological activities.
For the second module (Fig. 4c), diterpenoid synthetases (diTPSs) play a role in the formation of diterpene backbones in plant A. paniculata, which can be divided into two categories (class I and class II) following conserved modular structure (α, β and γ) within the proteins. Functionally, class II diTPSs (CPSs) initiate the cyclization of GGPP by protonation of a double bond into several bicyclic prenyl diphosphates with specific stereo configuration ((+)-CPP, ent-CPP), which are subsequently converted by class I diTPSs (KSLs) into labdane-related diterpenes (LRDs). Three class II diTPSs (ApCPS1, ApCPS2, and ApCPS3) probably participate in distinct diterpenoid metabolic pathways of A. paniculate[2]. ApCPS2 potentially converts GGPP to ent-CPP, as shown by biochemical characterization of the recombinant protein and accumulation of tissue-specific ent-LRDs[80]. While ApCPS1 has been demonstrated to produce both ent-CPP and (+)-CPP, ApCPS3 can only make (+)-CPP of normal stereochemistry as shown by GC-MS chromatograms of extracts from cultures expressing these proteins in E. coli also engineered to produced GGPP[2]. Moreover, two KSLs (ApKSL1 and ApKSL2) have been just recognized to consume ent-CPP and (+)-CPP but are not related to producing the ent-copalol or ent-labdatriene, the precursors of andrographolide and derived diterpenoids[2].
In addition, specific functional modifications of LRDs primarily include the addition of hydroxyl group and glycosylation, which can be mediated by the activities of cytochrome P450 monooxygenases (CYPs) and glycosyltransferases (GTs) individually (Fig. 4d). Furthermore, multi-omics data integration revealed some genes encoding CYPs, which may participate in the biosynthesis of andrographolide and related diterpenoids without being proved by functional studies[2]. And, ApUGTs are known as glycosyltransferases which are responsible for the formation of neoandrographolide, a glycosylated andrographinin. Recently, ApUGT12(UGT86C11) has been established to be closely involved in synthesizing neoandrographolide in A. paniculate[81]. Given the discovery of fewer key enzymes related to the biosynthetic pathway of andrographolide, there is still a need for further exploration and verification of the black box.
-
The medicinal characteristics of the plant A. paniculata are attributed to diterpene lactones and their derivatives, like andrographolide and neoandrographolide. Due to the limited availability of andrographolide from natural sources[82] and problems regarding the commercial cultivation of A. paniculata[83], it was proposed that biotechnological interventions may be exploited for the sustained production of andrographolide. Over the last 20 years, progress has been made in designing and constructing synthetic biology systems in yeast and bacteria, which serve as cell factories to produce second metabolites belonging to medical plants. Metabolic engineering in microorganisms can provide a cost‐effective alternative to gain medicinal diterpenoids such as forskolin[84]. However, heterologous production of andrographolide in microbes implies at least partial or total replication of the andrographolide biosynthetic pathway, which is not fully elucidated in A. paniculata. Fortunately, the production of diterpenoids and derivatives from other medicinal plants in microbial cell factories has been achieved, including the precursor of taxol (taxadiene)[85], tanshinone analogs (miltiradiene)[86], sclareol[87], etc. Thus, the strategies and methods of constructing cell factories to produce these diterpenes can be learned when we need to utilize microbes for the green production of andrographolide.
Corresponding to the original diterpenoid biosynthetic pathway in plants, diterpenoid cell factory construction is divided into three modules: upstream pathway enhancement (Fig. 4b), midstream pathway simulation, and downstream pathway exploration. In terms of upstream pathway enhancement, systematic multivariate analysis has been used to determine a balance between the IPP production and the skeleton formation, which could maximize the productive capacity of taxadiene[85]. This optimization strategy resulted in an E. coli strain containing an additional copy of the upstream pathway (to create isoprenoid precursors, IPP, and DMAPP) under the control of Trc promoter producing 2000-fold more taxadiene titers than those only expressing the native MEP pathway. Furthermore, to enlarge the endogenous precursor pools of GGPPS substrates (IPP and DMAPP), the copy number of rate-limiting steps (DXS, IDI, ISPD, ISPF) in the MEP pathway was amplified to five by additional expression, generating a 600-fold increase of levopimaradiene[88]. Notably, central metabolism and transcription factors have been manipulated in Saccharomyces cerevisiae to further rewire the global cellular metabolism for enhancing the supply of acetyl-CoA and NADPH, which resulted in a 160-fold increase in the production of sclareol[87].
For the midstream modules that form the diterpenoid skeleton, the construction of cell factories has focused on simulating diterpene synthases in plants. Extensive screening of diterpene synthases and their combinations from plants that can produce diterpenoid skeletons may be an effective strategy to improve heterologous productivity in specific hosts[89]. The most efficient combination of Coleus forskohlii TPS1 and Salvia miltiorrhiza KSL1 has finally been selected from the enzyme library for the high-level production of miltiradiene[89]. Besides, 51 functional combinations of class I and II diTPSs have been constructed in yeast by imitating the modularity of diterpenoid biosynthesis in plants, generating diverse diterpene skeletons[90]. Notably, the combination of diterpene synthases TwTPS9 and TwTPS27 from Tripterygium wilfordii has been introduced into yeast to produce the diterpene dehydroabietic acid, an intermediate of triptolide. Furthermore, the chloroplast transit peptide-truncated TwTPS9 and TwTPS27 raised the production of miltiradiene by about 7.1 fold[91]. Similarly, the fused expression of SmCPS and SmKSL led to a 2.9-fold increase in miltiradiene production by shortening the distance and avoiding the block between two active sites[92], which is inspired by the fact that the class II and class I diterpene synthases in plants commonly form complexes in plastids.
The conversion process from a diterpenoid skeleton to various compounds with specific pharmacological activities primarily occurred in the downstream modification module. Nevertheless, this module is unknown in most diterpenoid-producing plants. Therefore, microbial cell factories are used not only for metabolite production but also for identifying critical enzymes. Six CYPs have been examined in high-titer casbene-producing yeast strain JWY509 to verify the ability to produce jolkinol C. The optimal combination of JcCYP71D495 (C9OX2) and JcCYP726A20 (C5OX2) from Jatropha curcas has been selected to yield 400 mg/L jolkinol C[93]. In addition, two tandemly duplicated CYP82Ds from T. wilfordii have been found to catalyze the aromatization of miltiradiene in S. cerevisiae and successfully produced triptolide precursor 14-hydroxy-dehydroabietic acid[94]. Compared with diversification mediated by P450 oxidation, glycosylated diterpenoids in plants are relatively less. GjUGT94E13 and GjUGT74F8 from Gardenia jasminoides have been functionally characterized to catalyze the glycosylation of crocetin by using an E. coli cell factory[95]. Moreover, the classic case extensively studied is steviol glycosides (SGs). Based on the understanding that the crystal structure of the SrUGT76G1 (Stevia rebaudiana) can be used to accommodate various steviol-derived ligands, the substrate-binding pockets of EUGT11, SrUGT76G1, and UGTSL2 have been modified to convert stevioside/rebaudioside A to sweeter rebaudioside D/M[96,97].
-
A. paniculata is a well-known traditional medicinal herb that is utilized in India, China, Thailand, and Malaysia to treat infections and inflammation. It is widely distributed in Southeast and South Asia. According to the theory of TCM, it is intensely bitter in taste and cold-natured. Modern pharmacological research has discovered that the plant possesses various medicinal properties such as anti-tumor, anti-inflammatory, anti-viral, cardio-protecting, neuro-protectant, and hepato-protectant. Although andrographolide has been developed into many dosage forms for versatile applications in clinical treatment, we still need to pay more attention to the safety of andrographolide preparations, especially injection (which accounts for about 4.4% of the market). Since Chinese Food and Drug Administration (CFDA) has been warning of the severe allergic reactions after injecting 'Xi Yan Ping' in adults and forbidden using 'Yan Hu Ning' injections in children. Thus, future directions for developing pharmaceutical preparations need more extensive and comprehensive pharmacological experimental evidence and a clear mechanism and target organ of andrographolide.
Notably, andrographolide and related diterpene lactones have been identified as the primary therapeutic active component. Due to the high demand for andrographolide and its semi-synthetic derivatives, A. paniculata is commercially cultivated worldwide. Limited by the low content of andrographolide (3.8–31.2 mg/g DCW) and low plant growth rate, the price of purified andrographolide and its derivatives is about $100,000/kg[98]. Chemical synthesis of andrographolide under laboratory conditions is inefficient due to its complex structure and specific stereo-conformation. Therefore, it is imperative to adopt other biotechnological tools to produce andrographolide efficiently. However, the biosynthetic pathway of andrographolide has not been fully elucidated. Urgent research is focused on understanding the enzymatic process involved in the formation of andrographolide and the complete pathways. High‐throughput approaches, including multi-omics and comparative omics analysis combined with computational tools and artificial intelligence (AI), are excellent ways to narrow down the gene candidates from large gene families. Meanwhile, heterologous expression systems (stable expression in microbes and transient expression in tobacco leaves[99]) can provide diverse platforms for functionalizing key enzymes and act as green factories to produce andrographolide efficiently. In conclusion, bioreactor technologies can rapidly scale up andrographolide production by constructing cell factories with a complete understanding of the other diterpenoids' biosynthetic pathways.
-
The authors confirm contribution to the paper as follows: study conception and design: Zhang L, Chen R, Chen X; data collection and analysis: Chen X, Ren J, Yang J, Zhu Z; draft manuscript preparation: Chen X, Ren J, Yang J, Zhu Z, Chen R. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
We appreciate Huadong Li (Shanghai Kangqiao Chinese Medicine Tablet Co., Ltd) for photos of Andrographis paniculate. We also thank Luyao Yu (Naval Medical University, Shanghai, China) for discussing andrographolide bioactivities. This work was supported by the National Key Research and Development Program of China (No. 2022YFC3501703), the National Natural Science Foundation of China (No. 32000231, 32170274, 31970316, 82225047), and the Postgraduate Innovation Fund of Interdiscipline and New Medicine from School of Medicine of Shanghai University (China).
-
The authors declare that they have no conflict of interest. Lei Zhang is the Editorial Board member of Medicinal Plant Biology who was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and the research groups.
-
# These authors contributed equally: Xianghui Chen, Junze Ren, Jindong Yang, Zhanpin Zhu
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Chen X, Ren J, Yang J, Zhu Z, Chen R, et al. 2023. A critical review of Andrographis paniculata. Medicinal Plant Biology 2:15 doi: 10.48130/MPB-2023-0015
A critical review of Andrographis paniculata
- Received Date: 11 May 2023
- Accepted Date: 11 September 2023
- Published Online: 31 October 2023
Abstract: Andrographis paniculata, a traditional medicinal plant, is widely used to treat various disorders. According to traditional Chinese medicine (TCM) theory, it is intensely bitter in taste and cold-natured. Andrographolide and its derivates are the major bioactive compounds that show anti-inflammatory, anti-tumor, anti-diabetes, cardiovascular protection, neuroprotective, and hepatoprotective effects. In this review, we will focus on the application of TCM prescriptions and the modern bioactivities of Andrographis Herba. Due to the low content of andrographolide and derived lactones in the original species and the complexity of chemical structure, there is an urgent need to develop biotechnological methods for obtaining andrographolide and its derivatives sustainably. Nevertheless, the andrographolide biosynthetic pathway still needs to be fully elucidated in A. paniculata. Therefore, we further review recent progress in revealing the andrographolide biosynthetic pathway, combined with heterologous synthesis strategies of other plant diterpenoids to help create a cell factory with higher production of andrographolide in the future.