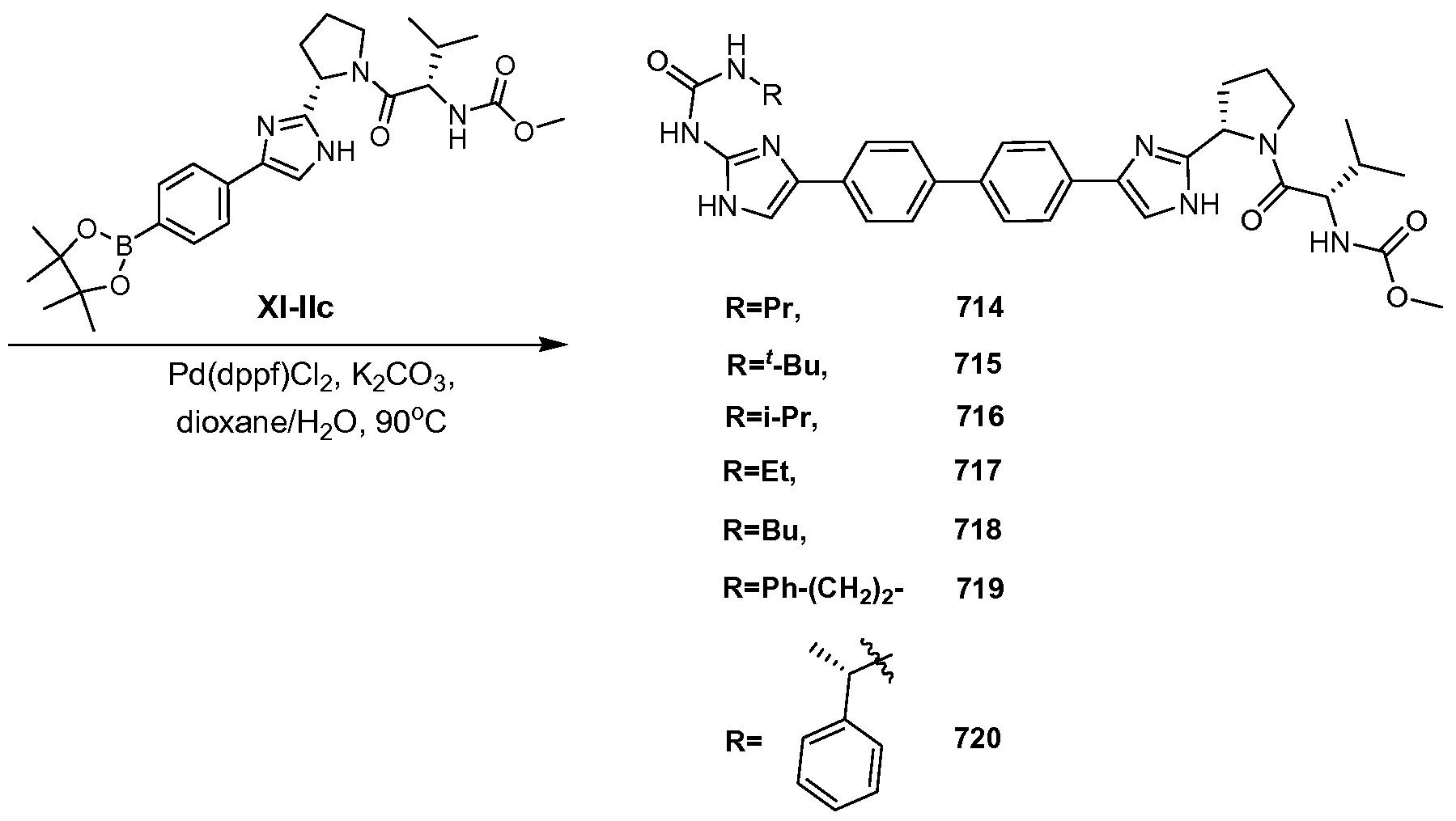
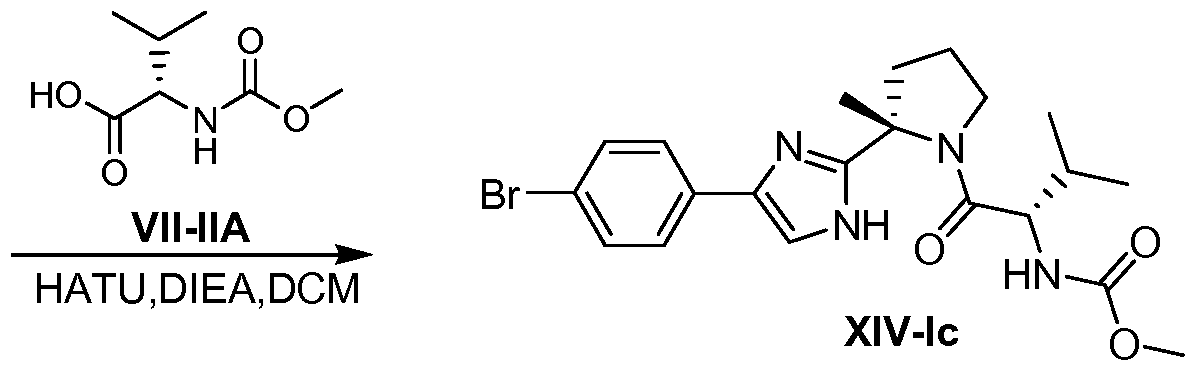
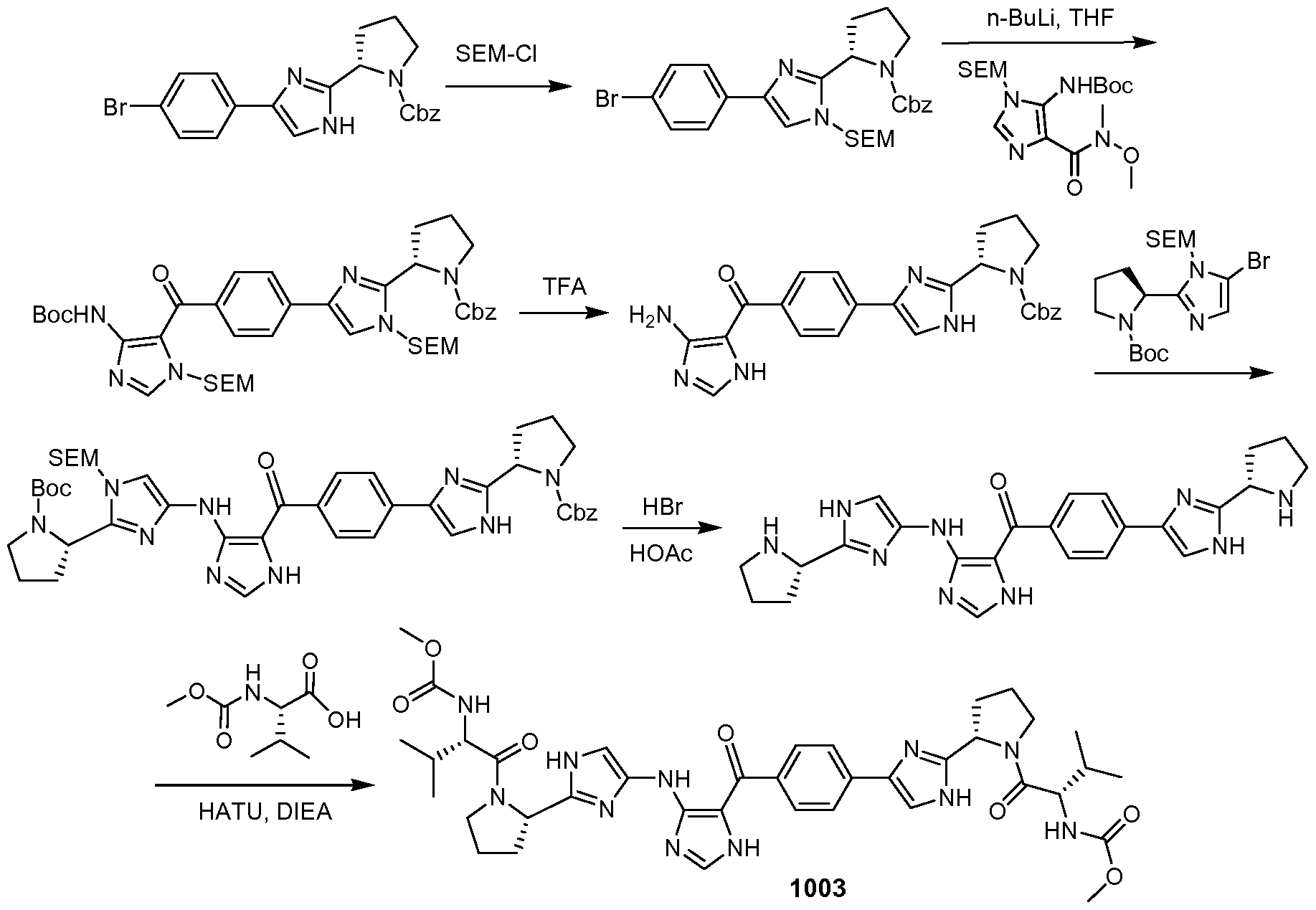
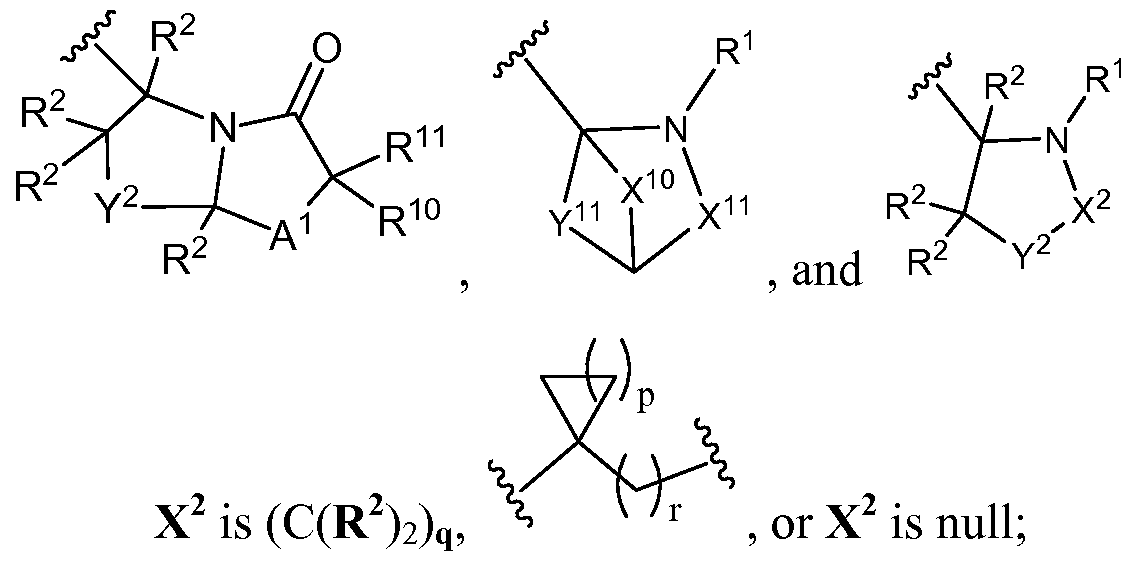WO2012087976A2 - Novel inhibitors of hepatitis c virus replication - Google Patents
Novel inhibitors of hepatitis c virus replication Download PDFInfo
- Publication number
- WO2012087976A2 WO2012087976A2 PCT/US2011/065925 US2011065925W WO2012087976A2 WO 2012087976 A2 WO2012087976 A2 WO 2012087976A2 US 2011065925 W US2011065925 W US 2011065925W WO 2012087976 A2 WO2012087976 A2 WO 2012087976A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- alkyl
- optionally substituted
- group
- halo
- aryl
- Prior art date
Links
- 0 C*(C=*1)C=C1c(cc1)ccc1-c(cc1)ccc1C1=C*(C)C(*2CCCC2)=*1 Chemical compound C*(C=*1)C=C1c(cc1)ccc1-c(cc1)ccc1C1=C*(C)C(*2CCCC2)=*1 0.000 description 11
- KYSGKLGXIPVPGQ-ZANVPECISA-N CC(C)[C@@H](C(N(CCC1)[C@]1(C)C(O)=O)=O)NC(OC)=O Chemical compound CC(C)[C@@H](C(N(CCC1)[C@]1(C)C(O)=O)=O)NC(OC)=O KYSGKLGXIPVPGQ-ZANVPECISA-N 0.000 description 2
- SIYGMNHVYBBURA-FMWQYVROSA-N CC(C)[C@@H](C(N(CCC1)[C@]1(C)C(OCC(c(cc1)ccc1-c(cc1)ccc1C(COC([C@](C)(CCC1)N1C([C@H](C(C)C)NC(OC)=O)=O)=O)=O)=O)=O)=O)NC(OC)=O Chemical compound CC(C)[C@@H](C(N(CCC1)[C@]1(C)C(OCC(c(cc1)ccc1-c(cc1)ccc1C(COC([C@](C)(CCC1)N1C([C@H](C(C)C)NC(OC)=O)=O)=O)=O)=O)=O)=O)NC(OC)=O SIYGMNHVYBBURA-FMWQYVROSA-N 0.000 description 2
- BSKOLJVTLRLTHE-UHFFFAOYSA-N CCC1C(C)CCC1 Chemical compound CCC1C(C)CCC1 BSKOLJVTLRLTHE-UHFFFAOYSA-N 0.000 description 2
- IONRYADUAUEBMP-UHFFFAOYSA-N COC(Nc1ncc(-c(cc2)ccc2Br)[nH]1)=O Chemical compound COC(Nc1ncc(-c(cc2)ccc2Br)[nH]1)=O IONRYADUAUEBMP-UHFFFAOYSA-N 0.000 description 2
- RTSLQVZQORGDQQ-UHFFFAOYSA-N O=C(CBr)c(cc1)ccc1-c(cc1)ccc1C(CBr)=O Chemical compound O=C(CBr)c(cc1)ccc1-c(cc1)ccc1C(CBr)=O RTSLQVZQORGDQQ-UHFFFAOYSA-N 0.000 description 2
- LPBDRMXUVIXKMF-UHFFFAOYSA-N CC(C)(C)OC([n]1c(N(C(OC)=O)C(OC)=O)ncc1-c(cc1)ccc1Br)=O Chemical compound CC(C)(C)OC([n]1c(N(C(OC)=O)C(OC)=O)ncc1-c(cc1)ccc1Br)=O LPBDRMXUVIXKMF-UHFFFAOYSA-N 0.000 description 1
- PDUSWJORWQPNRP-UHFFFAOYSA-N CC(C)NC(C)=O Chemical compound CC(C)NC(C)=O PDUSWJORWQPNRP-UHFFFAOYSA-N 0.000 description 1
- XRMJOPHPBRTRLE-SVBPBHIXSA-N CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2-c(cc2)ccc2-c2c[nH]c(NC(C)=O)n2)c[nH]1)=O)NC(NC)=O Chemical compound CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2-c(cc2)ccc2-c2c[nH]c(NC(C)=O)n2)c[nH]1)=O)NC(NC)=O XRMJOPHPBRTRLE-SVBPBHIXSA-N 0.000 description 1
- JNZVAZOLOXRSLB-UIOOFZCWSA-N CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2-c(cc2)ccc2-c2c[nH]c(NC(OC)=O)n2)c[nH]1)=O)NC(OC)=O Chemical compound CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2-c(cc2)ccc2-c2c[nH]c(NC(OC)=O)n2)c[nH]1)=O)NC(OC)=O JNZVAZOLOXRSLB-UIOOFZCWSA-N 0.000 description 1
- BHLPGQSNBFUPQA-FMYROPPKSA-N CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2-c(cc2)ccc2-c2c[nH]c([C@H]3NCCC3)n2)c[nH]1)=O)NC(OC)=O Chemical compound CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2-c(cc2)ccc2-c2c[nH]c([C@H]3NCCC3)n2)c[nH]1)=O)NC(OC)=O BHLPGQSNBFUPQA-FMYROPPKSA-N 0.000 description 1
- XIPXJGQTMSTAGN-DZXSPZCNSA-N CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2C(c2c(Nc3c[nH]c([C@H](CCC4)N4C([C@H](C(C)C)NC(OC)=O)=O)n3)nc[nH]2)=O)c[nH]1)=O)NC(OC)=O Chemical compound CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2C(c2c(Nc3c[nH]c([C@H](CCC4)N4C([C@H](C(C)C)NC(OC)=O)=O)n3)nc[nH]2)=O)c[nH]1)=O)NC(OC)=O XIPXJGQTMSTAGN-DZXSPZCNSA-N 0.000 description 1
- VNINAWXBULHDJF-DZXSPZCNSA-N CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2C(c2c(Nc3c[nH]c([C@H](CCC4)N4C([C@H](C(C)C)NC(OC)=O)=O)n3)nc[o]2)=O)c[nH]1)=O)NC(OC)=O Chemical compound CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2C(c2c(Nc3c[nH]c([C@H](CCC4)N4C([C@H](C(C)C)NC(OC)=O)=O)n3)nc[o]2)=O)c[nH]1)=O)NC(OC)=O VNINAWXBULHDJF-DZXSPZCNSA-N 0.000 description 1
- KZZXMKYQXDBLIK-DZXSPZCNSA-N CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2C(c2c(Nc3c[nH]c([C@H](CCC4)N4C([C@H](C(C)C)NC(OC)=O)=O)n3)nc[s]2)=O)c[nH]1)=O)NC(OC)=O Chemical compound CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2C(c2c(Nc3c[nH]c([C@H](CCC4)N4C([C@H](C(C)C)NC(OC)=O)=O)n3)nc[s]2)=O)c[nH]1)=O)NC(OC)=O KZZXMKYQXDBLIK-DZXSPZCNSA-N 0.000 description 1
- RMHVSHFUZWOLKS-MKJGCKHTSA-N CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2C(c2ccccc2Nc2c[nH]c([C@H](CCC3)N3C([C@H](C(C)C)NC(OC)=O)=O)n2)=O)c[nH]1)=O)NC(OC)=O Chemical compound CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2C(c2ccccc2Nc2c[nH]c([C@H](CCC3)N3C([C@H](C(C)C)NC(OC)=O)=O)n2)=O)c[nH]1)=O)NC(OC)=O RMHVSHFUZWOLKS-MKJGCKHTSA-N 0.000 description 1
- VFHSFXAFABSNRS-SFTDATJTSA-N CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(-c2ccc(B3OC(C)(C)C(C)(C)O3)cc2)c[nH]1)=O)NC(OC)=O Chemical compound CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(-c2ccc(B3OC(C)(C)C(C)(C)O3)cc2)c[nH]1)=O)NC(OC)=O VFHSFXAFABSNRS-SFTDATJTSA-N 0.000 description 1
- RLFKPITZAHNWPL-GSDHBNRESA-N CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(C(c2c(Nc(cc3)ccc3-c3c[nH]c([C@H](CCC4)N4C(C)=O)n3)[s]cn2)=O)c[nH]1)=O)NCO Chemical compound CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(C(c2c(Nc(cc3)ccc3-c3c[nH]c([C@H](CCC4)N4C(C)=O)n3)[s]cn2)=O)c[nH]1)=O)NCO RLFKPITZAHNWPL-GSDHBNRESA-N 0.000 description 1
- NHXGNXHXLRKJIL-DZUOILHNSA-N CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(C(c2c(Nc(cc3)ccc3-c3c[nH]c([C@H](CCC4)N4C([C@H](C(C)C)NC(OC)=O)=O)n3)nc[s]2)=O)c[nH]1)=O)NC(OC)=O Chemical compound CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(C(c2c(Nc(cc3)ccc3-c3c[nH]c([C@H](CCC4)N4C([C@H](C(C)C)NC(OC)=O)=O)n3)nc[s]2)=O)c[nH]1)=O)NC(OC)=O NHXGNXHXLRKJIL-DZUOILHNSA-N 0.000 description 1
- MXPCFFJYDZPUNS-XEZODYMFSA-N CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(Nc2c(C(c(cc3)ccc3-c3c[nH]c([C@H](CCC4)N4C([C@H](C(C)C)NC(OI)=O)=O)n3)=O)nc[o]2)c[nH]1)=O)NC(OC)=O Chemical compound CC(C)[C@@H](C(N(CCC1)[C@@H]1c1nc(Nc2c(C(c(cc3)ccc3-c3c[nH]c([C@H](CCC4)N4C([C@H](C(C)C)NC(OI)=O)=O)n3)=O)nc[o]2)c[nH]1)=O)NC(OC)=O MXPCFFJYDZPUNS-XEZODYMFSA-N 0.000 description 1
- WTGFPHJJWROJCK-GPSKOVRPSA-N CC(C)[C@@H](C(N(CCC1)[C@]1(C)C(NC(C)Br)=N)=O)NC(OC)=O Chemical compound CC(C)[C@@H](C(N(CCC1)[C@]1(C)C(NC(C)Br)=N)=O)NC(OC)=O WTGFPHJJWROJCK-GPSKOVRPSA-N 0.000 description 1
- BVXUPGLUJIJFBG-VVMGEOGYSA-N CC(C)[C@@H](C(N(CCC1)[C@]1(C)C(N[C@@H](C)C(C)c1c[nH]c([C@](C)(CCC2)N2C([C@H](C(C)C)NC(OC)=O)=O)n1)=N)=O)NC(OC)=O Chemical compound CC(C)[C@@H](C(N(CCC1)[C@]1(C)C(N[C@@H](C)C(C)c1c[nH]c([C@](C)(CCC2)N2C([C@H](C(C)C)NC(OC)=O)=O)n1)=N)=O)NC(OC)=O BVXUPGLUJIJFBG-VVMGEOGYSA-N 0.000 description 1
- BTCJRQPLMLTRII-UKZAINEFSA-N CC(C)[C@@H](C(N(CCC1)[C@]1(C)C(OCC(C(CC1C)=CC=C1c(cc1)ccc1C(COC([C@H](CCC1)N1C([C@H](C(C)C)NC(OC)=O)=O)=O)=O)=O)=O)O)NC(OC)=O Chemical compound CC(C)[C@@H](C(N(CCC1)[C@]1(C)C(OCC(C(CC1C)=CC=C1c(cc1)ccc1C(COC([C@H](CCC1)N1C([C@H](C(C)C)NC(OC)=O)=O)=O)=O)=O)=O)O)NC(OC)=O BTCJRQPLMLTRII-UKZAINEFSA-N 0.000 description 1
- CEFVHPDFGLDQKU-YFKPBYRVSA-N CC(C)[C@@H](C(O)=O)NC(OC)=O Chemical compound CC(C)[C@@H](C(O)=O)NC(OC)=O CEFVHPDFGLDQKU-YFKPBYRVSA-N 0.000 description 1
- JZEQOIIPINFZAF-KWRMZWAXSA-N CC(C)[C@@H](CN(CCC1)[C@@H]1c1nc(-c(cc2)ccc2-c(cc2)ccc2-c2c[nH]c([C@H](CCC3)N3C([C@H](C(C)C)NC(OC)=O)=O)n2)c[nH]1)NC(OC)=O Chemical compound CC(C)[C@@H](CN(CCC1)[C@@H]1c1nc(-c(cc2)ccc2-c(cc2)ccc2-c2c[nH]c([C@H](CCC3)N3C([C@H](C(C)C)NC(OC)=O)=O)n2)c[nH]1)NC(OC)=O JZEQOIIPINFZAF-KWRMZWAXSA-N 0.000 description 1
- DNXVDGWBMPLCMI-UHFFFAOYSA-N CC(CC1)CC1I Chemical compound CC(CC1)CC1I DNXVDGWBMPLCMI-UHFFFAOYSA-N 0.000 description 1
- ZLZGHYMPBDASID-XDKWHASVSA-N CC(NC([C@@]1(C)NCCC1)=N)Br Chemical compound CC(NC([C@@]1(C)NCCC1)=N)Br ZLZGHYMPBDASID-XDKWHASVSA-N 0.000 description 1
- FHFSVDXYQFWKPC-UHFFFAOYSA-N CC1(C2)C2CCCC1 Chemical compound CC1(C2)C2CCCC1 FHFSVDXYQFWKPC-UHFFFAOYSA-N 0.000 description 1
- TYZSRLZKCZDMHO-VFZIDIBOSA-N CCCN(CC(N[C@@H](C)C(/C=C\C)NC(CN(CCC)C(OC(C)(C)C)=O)=N)=N)C(CNC(OC)=O)=O Chemical compound CCCN(CC(N[C@@H](C)C(/C=C\C)NC(CN(CCC)C(OC(C)(C)C)=O)=N)=N)C(CNC(OC)=O)=O TYZSRLZKCZDMHO-VFZIDIBOSA-N 0.000 description 1
- WJOODOQRLJPEEO-UHFFFAOYSA-N CCCN(Cc1nc(-c2ccc(B3OC(C)(C)C(C)(C)O3)cc2)c[nH]1)C(OC(C)(C)C)=O Chemical compound CCCN(Cc1nc(-c2ccc(B3OC(C)(C)C(C)(C)O3)cc2)c[nH]1)C(OC(C)(C)C)=O WJOODOQRLJPEEO-UHFFFAOYSA-N 0.000 description 1
- UGMJMNXOQZDJHI-ZDCRTTOTSA-N CCN(CC)C([C@H](CCC1)N1c1nc(-c(cc2)ccc2-c(cc2)ccc2-c2c[nH]c([C@H](CCC3)N3C([C@H](C(C)C)NC(OC)=O)=O)n2)c[nH]1)=O Chemical compound CCN(CC)C([C@H](CCC1)N1c1nc(-c(cc2)ccc2-c(cc2)ccc2-c2c[nH]c([C@H](CCC3)N3C([C@H](C(C)C)NC(OC)=O)=O)n2)c[nH]1)=O UGMJMNXOQZDJHI-ZDCRTTOTSA-N 0.000 description 1
- CPAYBDWXQITUGP-WHHDSKRTSA-N CC[C@@H](C(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2Nc2c(C(c3c[nH]c([C@H](CCC4)N4C([C@H](C(C)C)NC(OC)=O)=O)n3)=O)[s]cn2)c[nH]1)=O)NC Chemical compound CC[C@@H](C(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2Nc2c(C(c3c[nH]c([C@H](CCC4)N4C([C@H](C(C)C)NC(OC)=O)=O)n3)=O)[s]cn2)c[nH]1)=O)NC CPAYBDWXQITUGP-WHHDSKRTSA-N 0.000 description 1
- YNPRLKTXNVZRML-AWEZNQCLSA-N COC(NCC(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2Br)c[nH]1)=O)=O Chemical compound COC(NCC(N(CCC1)[C@@H]1c1nc(-c(cc2)ccc2Br)c[nH]1)=O)=O YNPRLKTXNVZRML-AWEZNQCLSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing three or more hetero rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/403—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil condensed with carbocyclic rings, e.g. carbazole
- A61K31/404—Indoles, e.g. pindolol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
- A61K31/4168—1,3-Diazoles having a nitrogen attached in position 2, e.g. clonidine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
- A61K31/4178—1,3-Diazoles not condensed 1,3-diazoles and containing further heterocyclic rings, e.g. pilocarpine, nitrofurantoin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/42—Oxazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/425—Thiazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/425—Thiazoles
- A61K31/428—Thiazoles condensed with carbocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D417/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00
- C07D417/14—Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulfur atoms as the only ring hetero atoms, not provided for by group C07D415/00 containing three or more hetero rings
Definitions
- HCV hepatitis C virus
- HCV infection is the most common chronic blood borne infection in the United States. Although the numbers of new infections have declined, the burden of chronic infection is substantial, with Centers for Disease Control estimates of 3.9 million (1.8%) infected persons in the United States.
- Chronic liver disease is the tenth leading cause of death among adults in the United States, and accounts for approximately 25,000 deaths annually, or approximately 1% of all deaths. Studies indicate that 40% of chronic liver disease is HCV-related, resulting in an estimated 8,000-10,000 deaths each year. HCV-associated end-stage liver disease is the most frequent indication for liver transplantation among adults.
- Antiviral therapy of chronic hepatitis C has evolved rapidly over the last decade, with significant improvements seen in the efficacy of treatment. Nevertheless, even with combination therapy using pegylated IFN-a plus ribavirin, 40%> to 50% of patients fail therapy; they are nonresponders or relapsers. These patients currently have no effective therapeutic alternative. In particular, patients who have advanced fibrosis or cirrhosis on liver biopsy are at significant risk of developing complications of advanced liver disease, including ascites, jaundice, variceal bleeding, encephalopathy, and progressive liver failure, as well as a markedly increased risk of hepatocellular carcinoma.
- HCV is an enveloped positive strand RNA virus in the Flaviviridae family.
- the single strand HCV RNA genome is believed to be approximately 9500 nucleotides in length and has a single open reading frame (ORF) encoding a single large polyprotein of about 3000 amino acids.
- ORF open reading frame
- this polyprotein is cleaved at multiple sites by cellular and viral proteases to produce the structural and non-structural (NS) proteins of the virus.
- NS structural and non-structural
- the generation of mature nonstructural proteins (NS2, NS3, NS4, NS4A, NS4B, NS5A, and NS5B) is believed to be effected by two viral proteases.
- the first viral protease is believed to cleave at the NS2-NS3 junction of the polyprotein.
- the second viral protease is believed to be a serine protease contained within the N-terminal region of NS3 (herein referred to as "NS3 protease").
- NS3 protease is believed to mediate all of the subsequent cleavage events at sites downstream relative to the position of NS3 in the polyprotein (i.e., sites located between the C-terminus of NS3 and the C-terminus of the polyprotein).
- NS3 protease exhibits activity both in cis, at the NS3-NS4 cleavage site, and in trans, for the remaining NS4A-NS4B, NS4B-NS5A, and NS5A-NS5B sites.
- the NS4A protein is believed to serve multiple functions, acting as a cofactor for the NS3 protease and possibly assisting in the membrane localization of NS3 and other viral replicase components.
- the formation of the complex between NS3 and NS4A may be necessary for NS3-mediated processing events and enhances proteolytic efficiency at all sites recognized by NS3.
- the NS3 protease also appears to exhibit nucleoside triphosphatase and RNA helicase activities.
- NS5B is believed to be an RNA-dependent RNA polymerase involved in the replication of HCV RNA.
- compounds that inhibit the action of NS5 A in viral replication are potentially useful for the treatment of HCV.
- Some embodiments provide a compound having the structure of Formula XV:
- each R lab is separately selected from the group consisting of -[(Y 14 )(C(R 2 ) 2 ) r (NR 2 ) s (C(R 2 ) 2 ) r ]-[Y 14 (C(R 2 ) 2 ) r (NR 2 ) s (C(R 2 ) 2 ) r ] s -(Y 14 ) s -R 80 , -[(Y 14 )(C(R 2 ) 2 ) r (NR 2 ) s (C(R 2 ) 2 ) r ]-Y 14 (C(R 2 ) 2 ) r O(C(R 2 ) 2 ) r -(Y 14 ) s -R 80 ,
- each R 80 is separately selected from the group consisting of hydrogen, alkoxyalkyl, Ci_ 6 alkyl, C 3 - 7 cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and (R e R f N)alkyl, said alkoxyalkyl, Ci_ 6 alkyl, C 3 _ 7 cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (R e R f N)alkyl are each optionally substituted with one or more R lac ;
- Q 7 is selected from the group consisting of ,
- Y 2 is selected from O (oxygen), S (sulfur), S(O), S0 2 , NR 2 , and C(R 2 ) 2 with the proviso that when X 2 is null Y 2 is C(R 2 ) 2 ;
- each X 10 is (C(R 2 ) 2 ) q ;
- each X 11 is separately selected from the group consisting of (C(R 2 ) 2 ) q
- each Y 11 is separately selected from the group consisting of -0(C(R 2 ) 2 ) n -, -S(C(R 2 ) 2 ) lake-, -S(0)(C(R 2 ) 2 ) n -, -S0 2 (C(R 2 ) 2 ) n -, -NR 2 (C(R 2 ) 2 ) lake-, and (C(R 2 ) 2 ) q ;
- each C(R 2a ) 2 is separately selected, wherein each R 2a is separately selected from the group consisting of hydrogen, Ci_ 6 alkyl optionally substituted with up to 9 halo, aryl(CH 2 ) n -, and heteroaryl(CH 2 ) n -, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_ 6 alkoxy optionally substituted with up to 9 halo and Ci_ 6 alkyl optionally substituted with up to 9
- each R 3a is separately selected from the group consisting of hydrogen, and optionally substituted Ci_ 6 alkyl;
- each R 4a R 4b N is separately selected, wherein R 4a and R 4b are each separately selected from the group consisting of hydrogen, optionally substituted Ci_ 6 alkyl, and aryl(CH 2 ) tenu-;
- each R 5a is separately selected from the group consisting of optionally substituted Ci_ 6 alkyl, and aryl(CH 2 ) tenu-;
- each R 6a is separately selected from the group consisting of optionally substituted Ci_ 6 alkyl, and aryl(CH 2 ) tenu-;
- each A 1 is separately selected from the group consisting of C 2 _ 6 alkenyl, Ci_ 6 alkyl, and -(CH 2 ) n -0-(CH 2 ) m -, each optionally substituted with one or more
- R 2b is selected from the group consisting of hydrogen, Ci_ 6 alkyl, C 2 _ 6 alkenyl, C 2 _ 6 alkynyl, C 3 _ 7 cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl; each R c is selected from the group consisting of hydrogen, Ci_ 6 alkyl, C 2 _ 6 alkenyl, C 2 - 6 alkynyl, C 3 - 7 cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl, said alkyl optionally substituted with R e R f N-, alkoxy, or Ci_6alkylS-;
- each R a R b N is separately selected, wherein R a and R b are each separately selected from the group consisting of hydrogen, C 2 _ 6 alkenyl, and Ci_ 6 alkyl;
- J 2 is aryl, heteroaryl, heterocyclyl, cycloalkyl, cycloalkenyl, or polycyclic hydrocarbon, each optionally substituted with one or more R 15 ;
- each A is separately selected from the group consisting of CR 3 and N (nitrogen);
- L 6 is selected from the group consisting of
- each X is separately selected from the group consisting of CR and N (nitrogen);
- each Y 4 is separately selected from the group consisting of C(R 4 ) 2 , NR 4 , O (oxygen), and S (sulfur);
- each X 9 is separately selected from the group consisting of CH and N (nitrogen);
- each Y 10 is separately selected from the group consisting of -CH 2 - and -
- each n separately is 0, 1 or 2;
- each p separately is 1, 2, 3 or 4;
- each q separately is 1, 2, 3, 4 or 5;
- each r separately is 0, 1, 2, 3, or 4;
- each s separately is 0 or 1 ;
- R 9a is selected from the group consisting of -NR 9b R 9c , -OR 9d , Ci_ Ci_ 6 alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
- R 9b is selected from the group consisting of hydrogen, Ci_ 6 alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
- R 9c is selected from the group consisting of Ci_ 6 alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
- R 9d is selected from the group consisting of Ci_ 6 alkyl optionally substituted with up to 9 halo, and optionally substituted aryl.
- the compound of Formula XV has the structure of Formula XV , or a pharmaceutically acceptable salt thereof.
- the compound of Formula XV has the structure:
- L 4 can be selected from the
- each B can separately be selected, wherein B can be a fused optionally substituted saturated or unsaturated three- to seven-membered carbocyclic ring or a fused optionally substituted saturated or unsaturated three- to seven-membered heterocyclic ring, each optionally substituted with one or more R 4 ;
- L 3 can be selected from the group consisting of H , -(NR 9 )-, O (oxygen), S (sulfur), and -CH 2 -;
- X 3 can be selected from the group consisting of NH, O (oxygen), and S (sulfur);
- each X s can be separately selected from the group consisting of -NH-, O (oxygen), S (sulfur), and -CH 2 -;
- each X 6 can be separately selected from the group consisting of N (nitrogen), and CR 8 ;
- L 4 can be selected from the
- R 6 can be Ci_ 6 alkyl optionally substituted with up to 9 halo.
- R 6 can be methyl.
- Some embodiments provide a compound having the structure of Formula XVI:
- each X 2 is (C(R 2 ) 2 ) q , , or X 2 is null;
- each Y 2 is selected from O (oxygen), S (sulfur), S(O), S0 2 , NR 2 , and C(R 2 ) 2 with the proviso that when X 2 is null Y 2 is C(R 2 ) 2 ;
- each X 10 is (C(R 2 ) 2 ) q ;
- each X 11 is separately selected from the group consisting of (C(R 2 ) 2 ) q , and
- each Y 11 is separately selected from the group consisting of -0(C(R 2 ) 2 ) n -, -S(C(R 2 ) 2 ) n -, -S(0)(C(R 2 ) 2 ) n -, -S0 2 (C(R 2 ) 2 ) n -, -NR 2 (C(R 2 ) 2 ) n -, and (C(R 2 ) 2 ) q ;
- each R 80 is separately selected from the group consisting of hydrogen, alkoxyalkyl, Ci_ 6 alkyl, C 3 _ 7 cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and (R e R f N)alkyl, said alkoxyalkyl, Ci_ 6 alkyl, C 3 _ 7 cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (R e R f N)alkyl are each optionally substituted with one or more R lac ;
- each R 10 is R c R d N-;
- each C(R 2a ) 2 is separately selected, wherein each R 2a is separately selected from the group consisting of hydrogen, Ci_ 6 alkyl optionally substituted with up to 9 halo, aryl(CH 2 ) lake-, and heteroaryl(CH 2 ) forum-, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_ 6 alkoxy optionally substituted with up to 9 halo and Ci_ 6 alkyl optionally substituted with up to 9
- each R 3a is separately selected from the group consisting of hydrogen, and optionally substituted Ci_ 6 alkyl;
- each R 4a R 4b N is separately selected, wherein R 4a and R 4b are each separately selected from the group consisting of hydrogen, optionally substituted Ci_ 6 alkyl, and aryl(CH 2 ) n -;
- each R 5a is separately selected from the group consisting of optionally substituted Ci_ 6 alkyl, and aryl(CH 2 ) tenu-;
- each R 6a is separately selected from the group consisting of optionally substituted Ci_ 6 alkyl, and aryl(CH 2 ) n -;
- each A 1 is separately selected from the group consisting of C 2 _ 6 alkenyl, Ci_ 6 alkyl, and -(CH 2 ) n -0-(CH 2 ) m -, each optionally substituted with one or more
- R 2b is selected from the group consisting of hydrogen, Ci_ 6 alkyl, C 2 _ 6 alkenyl, C 2 _ 6 alkynyl, C 3 _ 7 cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl;
- each R 2c is selected from the group consisting of hydrogen, Ci_ 6 alkyl, C 2 _ 6 alkenyl, C 2 _ 6 alkynyl, C 3 _ 7 cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl, said alkyl optionally substituted with R e R f N-, alkoxy, or Ci_ 6 alkylS-; each R ⁇ is separately selected, wherein R a and R b are each separately selected from the group consisting of hydrogen, C 2 _ 6 alkenyl, and Ci_ 6 alkyl;
- J 2 is aryl, heteroaryl, heterocyclyl, cycloalkyl, cycloalkenyl, or polycyclic hydrocarbon, each optionally substituted with one or more R 15 ;
- each A is separately selected from the group consisting of CR and N (nitrogen);
- L 6 is selected from the group consisting of
- L is selected from the group consisting of
- each X is separately selected from the group consisting of CR 4 and N (nitrogen);
- each Y 4 is separately selected from the group consisting of C(R 4 ) 2 , NR 4 , O (oxygen), and S (sulfur);
- each X 9 is separately selected from the group consisting of CH and N (nitrogen);
- each Y 10 is separately selected from the group consisting of -CH 2 - and -
- each m separately is 1 or 2;
- each n separately is 0, 1 or 2;
- each p separately is 1, 2, 3 or 4;
- each q separately is 1, 2, 3, 4 or 5;
- each r separately is 0, 1, 2, 3, or 4;
- R 9a is selected from the group consisting of -NR 9b R 9c , -OR 9d , Ci_ Ci_ 6 alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
- R 9b is selected from the group consisting of hydrogen, Ci_ 6 alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
- R 9c is selected from the group consisting of Ci_ 6 alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
- R 9d is selected from the group consisting of Ci_ 6 alkyl optionally substituted with up to 9 halo, and optionally substituted aryl.
- the compound of Formula XVI has the structure:
- R 6 can be methyl.
- the compound of Formula XVI has the structure of Formula XVIa:
- L 4 can be selected from the group consisting of
- each X 3 can separately be selected from the group consisting of NH, O (oxygen), and S (sulfur);
- each X s can separately be selected from the group consisting of -NH-, O (oxygen), S (sulfur), and -CH 2 -;
- each X 6 can separately be selected from the group consisting of N (nitrogen), and CR 8 ;
- each B can separately be selected, wherein B can be a fused optionally substituted saturated or unsaturated three- to seven-membered carbocyclic ring or a fused optionally substituted saturated or unsaturated three- to seven-membered heterocyclic ring, each optionally substituted with one or more R 4
- Some embodiments provide a compound having the structure of Formula XVII:
- Q 7 is selected from the group consisting of ⁇ n ⁇ v , and
- each X 2 is (C(R 2 ) 2 ) q , , or X is null; each Y 2 is selected from O (oxygen), S (sulfur), S(O), S0 2 , NR 2 , and C(R 2 ) 2 with the proviso that when X 2 is null Y 2 is C(R 2 ) 2 ;
- each X 10 is (C(R 2 ) 2 ) q ;
- each X 11 is separately selected from the group consisting of (C(R 2 ) 2 ) q , and
- each Y 11 is separately selected from the group consisting of -0(C(R 2 ) 2 ) n -,
- each R lab is separately selected from the group consisting of -[(Y 14 )(C(R 2 ) 2 ) r (NR 2 ) s (C(R 2 ) 2 ) r ]-[Y 14 (C(R 2 ) 2 ) r (NR 2 ) s (C(R 2 ) 2 ) r ] s -(Y 14 ) s -R 80 , -[(Y 14 )(C(R 2 ) 2 ) r (NR 2 ) s (C(R 2 ) 2 ) r ]-Y 14 (C(R 2 ) 2 ) r O(C(R 2 ) 2 ) r -(Y 14 ) s -R 80 ,
- each R 80 is separately selected from the group consisting of hydrogen, alkoxyalkyl, Ci_ 6 alkyl, C 3 - 7 cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and (R e R f N)alkyl, said alkoxyalkyl, Ci_ 6 alkyl, C 3 _ 7 cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (R e R f N)alkyl are each optionally substituted with one or more R lac ;
- each R 10 is R c R d N-;
- each C(R 2a ) 2 is separately selected, wherein each R 2a is separately selected from the group consisting of hydrogen, Ci_ 6 alkyl optionally substituted with up to 9 halo, aryl(CH 2 ) n -, and heteroaryl(CH 2 ) n -, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_ 6 alkoxy optionally substituted with up to 9 halo and Ci_ 6 alkyl optionally substituted with up to 9
- each R 3a is separately selected from the group consisting of hydrogen, and optionally substituted Ci_ 6 alkyl;
- each R 4a R 4b N is separately selected, wherein R 4a and R 4b are each separately selected from the group consisting of hydrogen, optionally substituted Ci_ 6 alkyl, and aryl(CH 2 ) n -;
- each R 5a is separately selected from the group consisting of optionally substituted Ci_ 6 alkyl, and aryl(CH 2 ) tenu-;
- each R 6a is separately selected from the group consisting of optionally substituted Ci_ 6 alkyl, and aryl(CH 2 ) n -;
- each A 1 is separately selected from the group consisting of C 2 _ 6 alkenyl, Ci_ 6 alkyl, and -(CH 2 ) n -0-(CH 2 ) m -, each optionally substituted with one or more
- R 2b is selected from the group consisting of hydrogen, Ci_ 6 alkyl, C 2 _ 6 alkenyl, C 2 - 6 alkynyl, C 3 _ 7 cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl;
- each R 2c is selected from the group consisting of hydrogen, Ci_ 6 alkyl, C 2 _ 6 alkenyl, C 2 - 6 alkynyl, C 3 _ 7 cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl, said alkyl optionally substituted with R e R f N-, alkoxy, or Ci_6alkylS-;
- each R a R b N is separately selected, wherein R a and R b are each separately selected from the group consisting of hydrogen, C 2 _ 6 alkenyl, and Ci_ 6 alkyl;
- each J 2 is phenyl optionally substituted with one or more R 15 ;
- each A is separately selected from the group consisting of CR 3 and N (nitrogen);
- L 6 is selected from the group consisting of
- L 7 is selected from the group consisting of
- each L is separately selected from the group consisting of
- each X 4 is separately selected from the group consisting of CR 4 and N
- each Y 4 is separately selected from the group consisting of C(R 4 ) 2 , NR 4 , O (oxygen), and S (sulfur);
- each X 9 is separately selected from the group consisting of CH and N (nitrogen);
- each Y 10 is separately selected from the group consisting of -CH 2 - and -
- each m separately is 1 or 2;
- each n separately is 0, 1 or 2;
- each p separately is 1, 2, 3 or 4;
- each q separately is 1, 2, 3, 4 or 5;
- each r separately is 0, 1, 2, 3, or 4;
- each s separately is 0 or 1 ;
- R 9a is selected from the group consisting of -NR 9b R 9c , -OR 9d , Ci_ Ci_ 6 alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
- R 9b is selected from the group consisting of hydrogen, Ci_ 6 alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
- R 9c is selected from the group consisting of Ci_ 6 alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
- R 9d is selected from the group consisting of Ci_ 6 alkyl optionally substituted with up to 9 halo, and optionally substituted aryl.
- L 4 can be selected from the consisting of
- the compound of Formula XVI has the structure:
- Some embodiments provide a compound having the structure of Formula XVIII:
- R 1 ⁇ / R 13 of Q 16 and Q 17 is not ⁇ ; each X 2 is (C(R 2 ) 2 ), , or X 2 is null;
- each Y 2 is selected from O (oxygen), S (sulfur), S(O), S0 2 , NR 2 , and C(R 2 ) 2 with the proviso that when X 2 is null Y 2 is C(R 2 ) 2 ;
- each X 10 is (C(R 2 ) 2 ) q ;
- each X 11 is separately selected from the group consisting of (C(R 2 ) 2 ) q , and
- each Y 11 is separately selected from the group consisting of -0(C(R 2 ) 2 ) n -, -S(C(R 2 ) 2 ) lake-, -S(0)(C(R 2 ) 2 ) n -, -S0 2 (C(R 2 ) 2 ) n -, -NR 2 (C(R 2 ) 2 ) lake-, and (C(R 2 ) 2 ) q ;
- each R lab is separately selected from the group consisting of -[(Y 14 )(C(R 2 ) 2 ) r (NR 2 ) s (C(R 2 ) 2 ) r ]-[Y 14 (C(R 2 ) 2 ) r (NR 2 ) s (C(R 2 ) 2 ) r ] s -(Y 14 ) s -R 80 , -[(Y 14 )(C(R 2 ) 2 ) r (NR 2 ) s (C(R 2 ) 2 ) r ]-Y 14 (C(R 2 ) 2 ) r O(C(R 2 ) 2 ) r -(Y 14 ) s -R 80 ,
- each R 80 is separately selected from the group consisting of hydrogen, alkoxyalkyl, Ci_ 6 alkyl, C 3 - 7 cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and (R e R f N)alkyl, said alkoxyalkyl, Ci_ 6 alkyl, C 3 _ 7 cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (R e R f N)alkyl are each optionally substituted with one or more R lac ;
- each R 10 is R c R d N-;
- each C(R 2a ) 2 is separately selected, wherein each R 2a is separately selected from the group consisting of hydrogen, Ci_ 6 alkyl optionally substituted with up to 9 halo, aryl(CH 2 ) n -, and heteroaryl(CH 2 ) n -, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_ 6 alkoxy optionally substituted with up to 9 halo and Ci_ 6 alkyl optionally substituted with up to 9
- each R 3a is separately selected from the group consisting of hydrogen, and optionally substituted Ci_ 6 alkyl;
- each R 4a R 4b N is separately selected, wherein R 4a and R 4b are each separately selected from the group consisting of hydrogen, optionally substituted Ci_ 6 alkyl, and aryl(CH 2 ) n -;
- each R 5a is separately selected from the group consisting of optionally substituted Ci_ 6 alkyl, and aryl(CH 2 ) tenu-;
- each R 6a is separately selected from the group consisting of optionally substituted Ci_ 6 alkyl, and aryl(CH 2 ) n -;
- each A 1 is separately selected from the group consisting of C 2 _ 6 alkenyl, Ci_ 6 alkyl, and -(CH 2 ) n -0-(CH 2 ) m -, each optionally substituted with one or more
- R 2b is selected from the group consisting of hydrogen, Ci_ 6 alkyl, C 2 _ 6 alkenyl, C 2 _ 6 alkynyl, C 3 _ 7 cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl;
- each R 2c is selected from the group consisting of hydrogen, Ci_ 6 alkyl, C 2 _ 6 alkenyl, C 2 _ 6 alkynyl, C 3 _ 7 cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl, said alkyl optionally substituted with R e R f N-, alkoxy, or Ci_6alkylS-;
- each R a R b N is separately selected, wherein R a and R b are each separately selected from the group consisting of hydrogen, C 2 _ 6 alkenyl, and Ci_ 6 alkyl;
- L 14 is -J n -J 2 -J 12 -;
- J 2 is aryl, heteroaryl, heterocyclyl, cycloalkyl, cycloalkenyl, or polycyclic hydrocarbon, each optionally substituted with one or more R 15 ;
- J 11 is C(R 4 ) 2 , NR 4 , O (oxygen), or S (sulfur);
- 1 6 is selected from the group consisting of
- each L 5 is separately selected from the group consisting of
- each A is separately selected from the group consisting of CR 3 and N (nitrogen);
- L 17 is selected from the group consisting of aryl, heteroaryl, heterocyclyl, cycloalkyl, cycloalkenyl, or polycyclic hydrocarbon, each optionally substituted with one or more R 15.
- L is selected from the group consisting of
- each X 4 is separately selected from the group consisting of CR 4 and N
- each Y 4 is separately selected from the group consisting of C(R 4 ) 2 , NR 4 , O (oxygen), and S (sulfur);
- each X 9 is separately selected from the group consisting of CH and N (nitrogen);
- each Y 10 is separately selected from the group consisting of -CH 2 - and -
- each m separately is 1 or 2;
- each n separately is 0, 1 or 2;
- each p separately is 1, 2, 3 or 4;
- each q separately is 1, 2, 3, 4 or 5;
- each r separately is 0, 1, 2, 3, or 4;
- each s separately is 0 or 1 ;
- R 9a is selected from the group consisting of -NR 9b R 9c , -OR 9d , Ci_ Ci_ 6 alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
- R 9b is selected from the group consisting of hydrogen, Ci_ 6 alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
- R 9c is selected from the group consisting of Ci_ 6 alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
- R 9d is selected from the group consisting of Ci_ 6 alkyl optionally substituted with up to 9 halo, and optionally substituted aryl.
- the compound of Formula XVIII has the structure of Formula XVIIIa:
- B can be aryl, heteroaryl, or cycloalkenyl, each optionally substituted with one or more R 15 ;
- B 11 can be a heterocyclyl, cycloalkyl, or polycyclic hydrocarbon, each optionally substituted with one or more R 15 ;
- each R 16 can separately be hydrogen or R 15 .
- B can be aryl, or heteroaryl
- J 11 can be NR 4 ;
- B can be phenyl
- each X 14 can separately be selected from the group consisting of CR 17 and N (nitrogen);
- Y 14 can be selected from the group consisting of NR 17 , O (oxygen), and S (sulfur);
- each R 17 can be separately hydrogen or R 15 .
- L 18 can be 3 ⁇ 4 X 9 * ;
- R 4 can be hydrogen
- the compound of Formula XVIII has the structure:
- Some embodiments provide a pharmaceutical composition comprising a pharmaceutically acceptable excipient and a compound of Formulas XV, XVI, XVII or XVIII.
- Some embodiments provide a method of treating HCV infection in an individual, the method comprising administering to the individual an effective amount of a compound of Formulas XV, XVI, XVII or XVIII or a pharmaceutical composition comprising a pharmaceutically acceptable excipient and a compound of Formulas XV, XVI, XVII or XVIII.
- Some embodiments provide a method of treating HCV infection in an individual, the method comprising administering to the individual an effective amount of a compound of Formulas XV, XVI, XVII or XVIII or a pharmaceutical composition comprising a pharmaceutically acceptable excipient and a compound of Formulas XV, XVI, XVII or XVIII
- the method further comprises identifying a subject suffering from a hepatitis C infection.
- Some embodiments provide a method of treating liver fibrosis in an individual, the method comprising administering to the individual an effective amount of a compound of Formulas XV, XVI, XVII or XVIII or a pharmaceutical composition comprising a pharmaceutically acceptable excipient and a compound of Formulas XV, XVI, XVII or XVIII.
- the method further comprises identifying a subject suffering from a hepatitis C infection.
- Some embodiments provide a method of increasing liver function in an individual having a hepatitis C virus infection, the method comprising administering to the individual an effective amount of a compound of Formulas XV, XVI, XVII or XVIII or a pharmaceutical composition comprising a pharmaceutically acceptable excipient and a compound of Formulas XV, XVI, XVII or XVIII.
- the method further comprises identifying a subject suffering from a hepatitis C infection. DETAILED DESCRIPTION
- the terms "individual,” “host,” “subject,” and “patient” are used interchangeably herein, and refer to a mammal, including, but not limited to, primates, including simians and humans.
- liver function refers to a normal function of the liver, including, but not limited to, a synthetic function, including, but not limited to, synthesis of proteins such as serum proteins (e.g., albumin, clotting factors, alkaline phosphatase, aminotransferases (e.g., alanine transaminase, aspartate transaminase), 5'- nucleosidase, ⁇ -glutaminyltranspeptidase, etc.), synthesis of bilirubin, synthesis of cholesterol, and synthesis of bile acids; a liver metabolic function, including, but not limited to, carbohydrate metabolism, amino acid and ammonia metabolism, hormone metabolism, and lipid metabolism; detoxification of exogenous drugs; a hemodynamic function, including splanchnic and portal hemodynamics; and the like.
- serum proteins e.g., albumin, clotting factors, alkaline phosphatase, aminotransferases (e.g., alanine transa
- sustained viral response refers to the response of an individual to a treatment regimen for HCV infection, in terms of serum HCV titer.
- a sustained viral response refers to no detectable HCV R A (e.g., less than about 500, less than about 200, or less than about 100 genome copies per milliliter serum) found in the patient's serum for a period of at least about one month, at least about two months, at least about three months, at least about four months, at least about five months, or at least about six months following cessation of treatment.
- Treatment failure patients generally refers to HCV- infected patients who failed to respond to previous therapy for HCV (referred to as “non- responders") or who initially responded to previous therapy, but in whom the therapeutic response was not maintained (referred to as “relapsers").
- the previous therapy generally can include treatment with IFN-a monotherapy or IFN-a combination therapy, where the combination therapy may include administration of IFN-a and an antiviral agent such as ribavirin.
- treatment refers to obtaining a desired pharmacologic and/or physiologic effect.
- the effect may be prophylactic in terms of completely or partially preventing a disease or symptom thereof and/or may be therapeutic in terms of a partial or complete cure for a disease and/or adverse affect attributable to the disease.
- Treatment covers any treatment of a disease in a mammal, particularly in a human, and includes: (a) preventing the disease from occurring in a subject which may be predisposed to the disease but has not yet been diagnosed as having it; (b) inhibiting the disease, i.e., arresting its development; and (c) relieving the disease, i.e., causing regression of the disease.
- alkyl refers to a branched or unbranched fully saturated acyclic aliphatic hydrocarbon group (i.e. composed of carbon and hydrogen containing no double or triple bonds). In some embodiments, alkyls may be substituted or unsubstituted. Alkyls include, but are not limited to, methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tertiary butyl, pentyl, hexyl, and the like, each of which may be optionally substituted in some embodiments.
- heteroalkyl refers to a branched or unbrached fully saturated acyclic aliphatic hydrocarbon group containing one or more heteroatoms in the carbon back bone (i.e., an alkyl group in which one or more carbon atoms is replaced with a heteroatom).
- heteroalkyls may be substituted or unsubstituted.
- Heteroalkyls include, but are not limited to, ethers, thioethers, and alkyl-amino-alkyls.
- halo refers to fluoro, chloro, bromo, or iodo.
- alkoxy refers to straight or branched chain alkyl radical covalently bonded to the parent molecule through an— O— linkage. In some embodiments, alkoxys may be substituted or unsubstituted. Examples of alkoxy groups include, but are not limited to, methoxy, ethoxy, propoxy, isopropoxy, butoxy, n-butoxy, sec-butoxy, t-butoxy and the like.
- alkenyl refers to a monovalent straight or branched chain radical of from two to twenty carbon atoms containing at least one carbon-carbon double bond including, but not limited to, 1-propenyl, 2-propenyl, 2- methyl-l-propenyl, 1-butenyl, 2-butenyl, and the like. In some embodiments, alkenyls may be substituted or unsubstituted.
- alkynyl refers to a monovalent straight or branched chain radical of from two to twenty carbon atoms containing at least one carbon-carbon triple bond including, but not limited to, 1-propynyl, 1-butynyl, 2-butynyl, and the like. In some embodiments, alkynyls may be substituted or unsubstituted
- aryl refers to a homocyclic aromatic radical having one ring, two appended rings, or multiple fused rings.
- aryl groups include, but are not limited to, phenyl, naphthyl, biphenyl, phenanthrenyl, naphthacenyl, and the like. In some embodiments, aryls may be substituted or unsubstituted.
- cycloalkyl refers to a saturated aliphatic ring system radical having three to twenty carbon atoms including, but not limited to, cyclopropyl, cyclopentyl, cyclohexyl, cycloheptyl, and the like. In some embodiments, cycloalkyls may be substituted or unsubstituted.
- cycloalkenyl refers to an aliphatic ring system radical having three to twenty carbon atoms having at least one carbon-carbon double bond in the ring.
- examples of cycloalkenyl groups include, but are not limited to, cyclopropenyl, cyclopentenyl, cyclohexenyl, cycloheptenyl, and the like.
- cycloalkenyls may be substituted or unsubstituted.
- polycycloalkyl refers to saturated aliphatic ring system radical having at least two rings that are fused with or without bridgehead carbons.
- examples of polycycloalkyl groups include, but are not limited to, bicyclo[4.4.0]decanyl, bicyclo[2.2.1]heptanyl, adamantyl, norbornyl, and the like.
- polycycloalkenyl refers to aliphatic ring system radical having at least two rings that are fused with or without bridgehead carbons in which at least one of the rings has a carbon-carbon double bond.
- examples of polycycloalkenyl groups include, but are not limited to, norbornylenyl, 1,1 '- bicyclopentenyl, and the like.
- polycyclic hydrocarbon refers to a ring system radical in which all of the ring members are carbon atoms. Polycyclic hydrocarbons can be aromatic or can contain less than the maximum number of non-cumulative double bonds. Examples of polycyclic hydrocarbon include, but are not limited to, naphthyl, dihydronaphthyl, indenyl, fluorenyl, and the like.
- heterocyclic or “heterocyclyl” or “heterocycloalkyl” used herein refers to a cyclic ring system radical having at least one non-aromatic ring in which one or more ring atoms are not carbon, namely heteroatom.
- Monocyclic “heterocyclic” or “heterocyclyl” moieties are non-aromatic.
- Bicyclic “heterocyclic” or “heterocyclyl” moieties include one non-aromatic ring wherein at least one heteroatom is present in a ring.
- Tricyclic "heterocyclic” or “heterocyclyl” moieties include at least one non-aromatic ring wherein at least one heteroatom is present in a ring.
- heterocyclic groups include, but are not limited to, morpholinyl, tetrahydrofuranyl, dioxolanyl, pyrolidinyl, oxazolyl, pyranyl, pyrrolyl, isoindoline and the like.
- heteroaryl refers to an aromatic ring system radical in which one or more ring atoms are not carbon, namely heteroatom, having one ring or multiple fused rings. In fused ring systems, the one or more heteroatoms may be present in only one of the rings.
- heteroaryl groups include, but are not limited to, benzothiazyl, benzoxazyl, quinazolinyl, quinolinyl, isoquinolinyl, quinoxalinyl, pyridinyl, pyrrolyl, oxazolyl, indolyl, and the like. In some embodiments, heteroaryls may be substituted or unsubstituted.
- heteroatom refers to, for example, oxygen, sulfur and nitrogen.
- arylalkyl refers to one or more aryl groups appended to an alkyl radical.
- arylalkyl groups include, but are not limited to, benzyl, phenethyl, phenpropyl, phenbutyl, and the like.
- arylalkyls may be substituted or unsubstituted, and can be substituted on either the aryl or alkyl portion or on both.
- cycloalkylalkyl refers to one or more cycloalkyl groups appended to an alkyl radical.
- examples of cycloalkylalkyl include, but are not limited to, cyclohexylmethyl, cyclohexylethyl, cyclopentylmethyl, cyclopentylethyl, and the like.
- cycloalkylalkyls may be substituted or unsubstituted.
- heteroarylalkyl refers to one or more heteroaryl groups appended to an alkyl radical.
- heteroarylalkyl include, but are not limited to, pyridylmethyl, furanylmethyl, thiopheneylethyl, and the like.
- heteroarylalkyls may be substituted or unsubstituted, and can be substituted on either the heteroaryl or alkyl portion or on both.
- heterocyclylalkyl refers to one or more heterocyclyl groups appended to an alkyl radical.
- heterocyclylalkyl include, but are not limited to, morpholinylmethyl, morpholinylethyl, morpholinylpropyl, tetrahydrofuranylmethyl, pyrrolidinylpropyl, and the like.
- aryloxy used herein refers to an aryl radical covalently bonded to the parent molecule through an— O— linkage.
- alkylthio refers to straight or branched chain alkyl radical covalently bonded to the parent molecule through an — S— linkage.
- alkylthio groups include, but are not limited to, methanesulfide, ethanesulfide, propanesulfide, isopropanesulfide, butanesulfide, n-butanesulfide, sec- butanesulfide, tert-butanesulfide and the like.
- arylthio refers to an aryl radical covalently bonded to the parent molecule through an— S— linkage.
- alkylamino refers to nitrogen radical with one or more alkyl groups attached thereto.
- monoalkylamino refers to nitrogen radical with one alkyl group attached thereto and dialkylamino refers to nitrogen radical with two alkyl groups attached thereto.
- cyanoamino used herein refers to nitrogen radical with nitrile group attached thereto.
- carboxy used herein refers to -COOH.
- sulfamyl used herein refers to -S0 2 NH 2 .
- thiocarboxy used herein refers to CSOH.
- sulfonamide used herein refers to -S0 2 NR' 2 where each R' is individually selected from H (hydrogen), Ci-C 6 alkyl, C 3 -C 7 cycloalkyl, arylalkyl and aryl optionally substituted with Ci-C 6 alkyl.
- esters used herein refers to -COOR' where R' is selected from Ci-C 6 alkyl, C 3 -C 7 cycloalkyl, arylalkyl and aryl optionally substituted with Ci-C 6 alkyl.
- a radical indicates a species with one or more, unpaired electron such that the species containing the radical can be covalently bonded to one or more other species.
- a radical is not necessarily a free radical. Rather, a radical indicates a specific portion of a larger molecule.
- the term "radical” can be used interchangeably with the term "moiety" or "group.”
- a substituted group is derived from the unsubstituted parent structure in which there has been an exchange of one or more hydrogen atoms for another atom or group.
- the substituent group(s) is (are) one or more group(s) individually and independently selected from Ci-C 6 alkyl, Ci-C 6 alkenyl, Ci-C 6 alkynyl, C3-C7 cycloalkyl (optionally substituted with halo, alkyl, alkoxy, carboxyl, haloalkyl, CN, -S0 2 -alkyl, -CF 3 , and -OCF 3 ), cycloalkyl geminally attached, Ci-C 6 heteroalkyl, C 3 -Ci 0 heterocycloalkyl (e.g., tetrahydrofuryl) (optionally substituted with halo, alkyl, alkoxy, carboxyl, CN, -S0 2 -alkyl, -CF 3 , and -OCF 3 ), aryl (optionally substituted with halo, alkyl, aryl optionally substituted with Ci-C 6 alkyl,
- Asymmetric carbon atoms may be present in the compounds described. All such isomers, including diastereomers and enantiomers, as well as the mixtures thereof are intended to be included in the scope of the recited compound. In certain cases, compounds can exist in tautomeric forms. All tautomeric forms are intended to be included in the scope. Likewise, when compounds contain an alkenyl or alkenylene group, there exists the possibility of cis- and trans- isomeric forms of the compounds. Both cis- and trans- isomers, as well as the mixtures of cis- and trans- isomers, are contemplated. Thus, reference herein to a compound includes all of the aforementioned isomeric forms unless the context clearly dictates otherwise.
- a polymorph is a composition having the same chemical formula, but a different structure.
- a solvate is a composition formed by solvation (the combination of solvent molecules with molecules or ions of the solute).
- a hydrate is a compound formed by an incorporation of water.
- a conformer is a structure that is a conformational isomer. Conformational isomerism is the phenomenon of molecules with the same structural formula but different conformations (conformers) of atoms about a rotating bond. Salts of compounds can be prepared by methods known to those skilled in the art.
- salts of compounds can be prepared by reacting the appropriate base or acid with a stoichiometric equivalent of the compound.
- a prodrug is a compound that undergoes biotransformation (chemical conversion) before exhibiting its pharmacological effects.
- a prodrug can thus be viewed as a drug containing specialized protective groups used in a transient manner to alter or to eliminate undesirable properties in the parent molecule.
- reference herein to a compound includes all of the aforementioned forms unless the context clearly dictates otherwise.
- pharmaceutically acceptable salt refers to any pharmaceutically acceptable salts of a compound, and preferably refers to an acid addition salt of a compound.
- pharmaceutically acceptable salts are acid addition salts of pharmaceutically acceptable inorganic or organic acids, including, but not limited to, hydrohalic, sulfuric, phosphoric, aliphatic or aromatic carboxylic, or sulfonic acid.
- Examples of pharmaceutically acceptable inorganic or organic acids as a component of an addition salt include but are not limited to, hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid acetic acid, succinic acid, lactic acid, malic acid, tartaric acid, citric acid, ascorbi acid c, nicotinic acid, methanesulfonic acid, p-toluensulfonic acid or naphthalenesulfonic acid acid.
- the preferred examples of pharmaceutically acceptable salts include, but are not limited to, alkali metal salts (sodium or potassium), alkaline earth metal salts (calcium or magnesium), or ammonium salts derived from ammonia or from pharmaceutically acceptable organic amines, for example C 1 -C7 alkylamine, cyclohexylamine, triethanolamine, ethylenediamine or tris- (hydroxymethyl)-aminomethane .
- Isotopes may be present in the compounds described. Each chemical element as represented in a compound structure may include any isotope of said element.
- a hydrogen atom may be explicitely disclosed or understood to be present in the compound.
- the hydrogen atom can be any isotope of hydrogen, including but not limited to hydrogen- 1 (protium) and hydrogen-2 (deuterium).
- reference herein to a compound encompasses all potential isotopic forms unless the context clearly dictates otherwise.
- a substituent as depicted as a di-radical i.e., has two points of attachment to the rest of the molecule
- the substituent can be attached in any directional configuration unless otherwise indicated.
- a substituent depicted as -AE- or 3 ⁇ 4 A ⁇ E A includes the substituent being oriented such that the A is attached at the leftmost attachment point of the molecule as well as the case in which A is attached at the rightmost attachment point of the molecule.
- radical naming conventions can include either a mono-radical or a di-radical, depending on the context. For example, where a substituent requires two points of attachment to the rest of the molecule, it is understood that the substituent is a di-radical.
- attachment includes di-radicals such as
- the present embodiments provide compounds of Formulas XV, XVI, XVII or XVIII, as defined above, as well as pharmaceutical compositions and formulations comprising any compound of Formulas XV, XVI, XVII or XVIII.
- a subject compound is useful for treating HCV infection and other disorders, as discussed below.
- a subject compound inhibits HCV viral replication.
- a subject compound inhibits HCV viral replication by at least about 10%, at least about 15%, at least about 20%>, at least about 25%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80%, or at least about 90%, or more, compared to HCV viral replication in the absence of the compound.
- Whether a subject compound inhibits HCV viral replication can be determined using methods known in the art, including an in vitro viral replication assay.
- compositions including pharmaceutical compositions, comprising compounds of the general Formulas XV, XVI, XVII or XVIII.
- a subject pharmaceutical composition comprises a subject compound; and a pharmaceutically acceptable excipient.
- a pharmaceutically acceptable excipient A wide variety of pharmaceutically acceptable excipients is known in the art and need not be discussed in detail herein. Pharmaceutically acceptable excipients have been amply described in a variety of publications, including, for example, A. Gennaro (2000) "Remington: The Science and Practice of Pharmacy," 20th edition, Lippincott, Williams, & Wilkins; Pharmaceutical Dosage Forms and Drug Delivery Systems (1999) H.C. Ansel et al, eds., 7 th ed., Lippincott, Williams, & Wilkins; and Handbook of Pharmaceutical Excipients (2000) A.H. Kibbe et al., eds., 3 rd ed. Amer. Pharmaceutical Assoc.
- compositions such as vehicles, adjuvants, carriers or diluents
- pharmaceutically acceptable auxiliary substances such as pH adjusting and buffering agents, tonicity adjusting agents, stabilizers, wetting agents and the like, are known in the art.
- a compound as described herein is formulated in an aqueous buffer.
- Suitable aqueous buffers include, but are not limited to, acetate, succinate, citrate, and phosphate buffers varying in strengths from about 5mM to about lOOmM.
- the aqueous buffer includes reagents that provide for an isotonic solution. Such reagents include, but are not limited to, sodium chloride; and sugars e.g., mannitol, dextrose, sucrose, and the like.
- the aqueous buffer further includes a non-ionic surfactant such as polysorbate 20 or 80.
- the formulations may further include a preservative.
- Suitable preservatives include, but are not limited to, a benzyl alcohol, phenol, chlorobutanol, benzalkonium chloride, and the like. In many cases, the formulation is stored at about 4°C. Formulations may also be lyophilized, in which case they generally include cryoprotectants such as sucrose, trehalose, lactose, maltose, mannitol, and the like. Lyophilized formulations can be stored over extended periods of time, even at ambient temperatures.
- administration of the compounds as described herein can be achieved in various ways, including oral, buccal, rectal, parenteral, intraperitoneal, intradermal, subcutaneous, intramuscular, transdermal, intratracheal, etc., administration.
- administration is by bolus injection, e.g., subcutaneous bolus injection, intramuscular bolus injection, and the like.
- compositions of the embodiments can be administered orally, parenterally or via an implanted reservoir. Oral administration or administration by injection is preferred.
- Subcutaneous administration of a pharmaceutical composition of the embodiments is accomplished using standard methods and devices, e.g., needle and syringe, a subcutaneous injection port delivery system, and the like. See, e.g., U.S. Patent Nos. 3,547,119; 4,755,173; 4,531,937; 4,311,137; and 6,017,328.
- a combination of a subcutaneous injection port and a device for administration of a pharmaceutical composition of the embodiments to a patient through the port is referred to herein as "a subcutaneous injection port delivery system.”
- subcutaneous administration is achieved by bolus delivery by needle and syringe.
- the compounds as described herein may be administered in the form of their pharmaceutically acceptable salts, or they may also be used alone or in appropriate association, as well as in combination, with other pharmaceutically active compounds.
- the following methods and excipients are merely exemplary and are in no way limiting.
- the compounds as described herein can be used alone or in combination with appropriate additives to make tablets, powders, granules or capsules, for example, with conventional additives, such as lactose, mannitol, corn starch or potato starch; with binders, such as crystalline cellulose, cellulose derivatives, acacia, corn starch or gelatins; with disintegrators, such as corn starch, potato starch or sodium carboxymethylcellulose; with lubricants, such as talc or magnesium stearate; and if desired, with diluents, buffering agents, moistening agents, preservatives and flavoring agents.
- conventional additives such as lactose, mannitol, corn starch or potato starch
- binders such as crystalline cellulose, cellulose derivatives, acacia, corn starch or gelatins
- disintegrators such as corn starch, potato starch or sodium carboxymethylcellulose
- lubricants such as talc or magnesium stearate
- the compounds as described herein can be formulated into preparations for injection by dissolving, suspending or emulsifying them in an aqueous or nonaqueous solvent, such as vegetable or other similar oils, synthetic aliphatic acid glycerides, esters of higher aliphatic acids or propylene glycol; and if desired, with conventional additives such as solubilizers, isotonic agents, suspending agents, emulsifying agents, stabilizers and preservatives.
- the compounds as described herein can be made into suppositories by mixing with a variety of bases such as emulsifying bases or water-soluble bases.
- bases such as emulsifying bases or water-soluble bases.
- the compounds of the embodiments can be administered rectally via a suppository.
- the suppository can include vehicles such as cocoa butter, carbowaxes and polyethylene glycols, which melt at body temperature, yet are solidified at room temperature.
- Unit dosage forms for oral or rectal administration such as syrups, elixirs, and suspensions may be provided wherein each dosage unit, for example, teaspoonful, tablespoonful, tablet or suppository, contains a predetermined amount of the composition containing one or more compounds as described herein.
- unit dosage forms for injection or intravenous administration may comprise the compounds as described herein in a composition as a solution in sterile water, normal saline or another pharmaceutically acceptable carrier.
- unit dosage form refers to physically discrete units suitable as unitary dosages for human and animal subjects, each unit containing a predetermined quantity of compounds of the embodiments calculated in an amount sufficient to produce the desired effect in association with a pharmaceutically acceptable diluent, carrier or vehicle.
- the specifications for the novel unit dosage forms of the embodiments depend on the particular compound employed and the effect to be achieved, and the pharmacodynamics associated with each compound in the host.
- compositions such as vehicles, adjuvants, carriers or diluents
- pharmaceutically acceptable auxiliary substances such as pH adjusting and buffering agents, tonicity adjusting agents, stabilizers, wetting agents and the like, are readily known in the art.
- Preferred embodiments provide a method of treating a hepatitis C virus infection in an individual, the method comprising administering to the individual an effective amount of a composition comprising a subject compound.
- Preferred embodiments provide a method of treating liver fibrosis in an individual, the method comprising administering to the individual an effective amount of a composition comprising a subject compound.
- Preferred embodiments provide a method of increasing liver function in an individual having a hepatitis C virus infection, the method comprising administering to the individual an effective amount of a composition comprising a subject compound.
- Whether a subject method is effective in treating an HCV infection can be determined by a reduction in viral load, a reduction in time to seroconversion (virus undetectable in patient serum), an increase in the rate of sustained viral response to therapy, a reduction of morbidity or mortality in clinical outcomes, or other indicator of disease response.
- an effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents is an amount that is effective to reduce viral load or achieve a sustained viral response to therapy.
- Whether a subject method is effective in treating an HCV infection can be determined by measuring viral load, or by measuring a parameter associated with HCV infection, including, but not limited to, liver fibrosis, elevations in serum transaminase levels, and necroinflammatory activity in the liver. Indicators of liver fibrosis are discussed in detail below.
- the methods involve administering an effective amount of a compound of Formulas XV, XVI, XVII or XVIII, optionally in combination with an effective amount of one or more additional antiviral agents.
- an effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents is an amount that is effective to reduce viral titers to undetectable levels, e.g., to about 1000 to about 5000, to about 500 to about 1000, or to about 100 to about 500 genome copies/mL serum.
- an effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents is an amount that is effective to reduce viral load to lower than 100 genome copies/mL serum.
- an effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents is an amount that is effective to achieve a 1.5-log, a 2-log, a 2.5-log, a 3-log, a 3.5-log, a 4-log, a 4.5-log, or a 5-log reduction in viral titer in the serum of the individual.
- an effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents is an amount that is effective to achieve a sustained viral response, e.g., non- detectable or substantially non-detectable HCV R A (e.g., less than about 500, less than about 400, less than about 200, or less than about 100 genome copies per milliliter serum) is found in the patient's serum for a period of at least about one month, at least about two months, at least about three months, at least about four months, at least about five months, or at least about six months following cessation of therapy.
- a sustained viral response e.g., non- detectable or substantially non-detectable HCV R A (e.g., less than about 500, less than about 400, less than about 200, or less than about 100 genome copies per milliliter serum) is found in the patient's serum for a period of at least about one month, at least about two months, at least about
- liver fibrosis As noted above, whether a subject method is effective in treating an HCV infection can be determined by measuring a parameter associated with HCV infection, such as liver fibrosis. Methods of determining the extent of liver fibrosis are discussed in detail below. In some embodiments, the level of a serum marker of liver fibrosis indicates the degree of liver fibrosis.
- ALT serum alanine aminotransferase
- an effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents is an amount effective to reduce ALT levels to less than about 45 IU/mL serum.
- a therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents is an amount that is effective to reduce a serum level of a marker of liver fibrosis by at least about 10%, at least about 20%, at least about 25%, at least about 30%, at least about 35%, at least about 40%), at least about 45%, at least about 50%>, at least about 55%, at least about 60%>, at least about 65%, at least about 70%>, at least about 75%, or at least about 80%, or more, compared to the level of the marker in an untreated individual, or to a placebo-treated individual.
- Methods of measuring serum markers include immunological-based methods, e.g., enzyme-linked immunosorbent assays (ELISA), radioimmunoassays, and the like, using antibody specific for a given serum marker.
- an effective amount of a compound of Formulas XV, XVI, XVII or XVIII and an additional antiviral agent is a synergistic amount.
- a "synergistic combination" or a "synergistic amount" of a compound of Formulas XV, XVI, XVII or XVIII and an additional antiviral agent is a combined dosage that is more effective in the therapeutic or prophylactic treatment of an HCV infection than the incremental improvement in treatment outcome that could be predicted or expected from a merely additive combination of (i) the therapeutic or prophylactic benefit of the compound of Formulas XV, XVI, XVII or XVIII when administered at that same dosage as a monotherapy and (ii) the therapeutic or prophylactic benefit of the additional antiviral agent when administered at the same dosage as a monotherapy.
- a selected amount of a compound of Formulas XV, XVI, XVII or XVIII and a selected amount of an additional antiviral agent are effective when used in combination therapy for a disease, but the selected amount of the compound of Formulas XV, XVI, XVII or XVIII and/or the selected amount of the additional antiviral agent is less effective when used in monotherapy for the disease.
- the embodiments encompass (1) regimens in which a selected amount of the additional antiviral agent enhances the therapeutic benefit of a selected amount of the compound of Formulas XV, XVI, XVII or XVIII when used in combination therapy for a disease, where the selected amount of the additional antiviral agent provides negligible therapeutic benefit when used in monotherapy for the disease (2) regimens in which a selected amount of the compound of Formulas XV, XVI, XVII or XVIII enhances the therapeutic benefit of a selected amount of the additional antiviral agent when used in combination therapy for a disease, where the selected amount of the compound of Formulas XV, XVI, XVII or XVIII provides negligible therapeutic benefit when used in monotherapy for the disease and (3) regimens in which a selected amount of the compound of Formulas XV, XVI, XVII or XVIII and a selected amount of the additional antiviral agent provide a therapeutic benefit when used in combination therapy for a disease, where each of the selected amount
- a "synergistically effective amount" of a compound of Formulas XV, XVI, XVII or XVIII and an additional antiviral agent, and its grammatical equivalents, shall be understood to include any regimen encompassed by any of (l)-(3) above.
- the embodiments provides methods for treating liver fibrosis (including forms of liver fibrosis resulting from, or associated with, HCV infection), generally involving administering a therapeutic amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents.
- Effective amounts of compounds of Formulas XV, XVI, XVII or XVIII, with and without one or more additional antiviral agents, as well as dosing regimens, are as discussed below.
- liver fibrosis reduction is determined by analyzing a liver biopsy sample.
- An analysis of a liver biopsy comprises assessments of two major components: necroinflammation assessed by "grade” as a measure of the severity and ongoing disease activity, and the lesions of fibrosis and parenchymal or vascular remodeling as assessed by "stage” as being reflective of long-term disease progression. See, e.g., Brunt (2000) Hepatol.
- METAVIR Hepatology 20: 15-20. Based on analysis of the liver biopsy, a score is assigned.
- the METAVIR scoring system is based on an analysis of various features of a liver biopsy, including fibrosis (portal fibrosis, centrilobular fibrosis, and cirrhosis); necrosis (piecemeal and lobular necrosis, acidophilic retraction, and ballooning degeneration); inflammation (portal tract inflammation, portal lymphoid aggregates, and distribution of portal inflammation); bile duct changes; and the Knodell index (scores of periportal necrosis, lobular necrosis, portal inflammation, fibrosis, and overall disease activity).
- each stage in the METAVIR system is as follows: score: 0, no fibrosis; score: 1, stellate enlargement of portal tract but without septa formation; score: 2, enlargement of portal tract with rare septa formation; score: 3, numerous septa without cirrhosis; and score: 4, cirrhosis.
- Knodell's scoring system also called the Hepatitis Activity Index, classifies specimens based on scores in four categories of histologic features: I. Periportal and/or bridging necrosis; II. Intralobular degeneration and focal necrosis; III. Portal inflammation; and IV. Fibrosis.
- scores are as follows: score: 0, no fibrosis; score: 1, mild fibrosis (fibrous portal expansion); score: 2, moderate fibrosis; score: 3, severe fibrosis (bridging fibrosis); and score: 4, cirrhosis. The higher the score, the more severe the liver tissue damage.
- the Scheuer scoring system scores are as follows: score: 0, no fibrosis; score: 1, enlarged, fibrotic portal tracts; score: 2, periportal or portal-portal septa, but intact architecture; score: 3, fibrosis with architectural distortion, but no obvious cirrhosis; score: 4, probable or definite cirrhosis. Scheuer (1991) J. Hepatol. 13:372.
- the Ishak scoring system is described in Ishak (1995) J. Hepatol. 22:696-699. Stage 0, No fibrosis; Stage 1, Fibrous expansion of some portal areas, with or without short fibrous septa; stage 2, Fibrous expansion of most portal areas, with or without short fibrous septa; stage 3, Fibrous expansion of most portal areas with occasional portal to portal (P-P) bridging; stage 4, Fibrous expansion of portal areas with marked bridging (P-P) as well as portal-central (P-C); stage 5, Marked bridging (P-P and/or P-C) with occasional nodules (incomplete cirrhosis); stage 6, Cirrhosis, probable or definite.
- the benefit of anti-fibrotic therapy can also be measured and assessed by using the Child-Pugh scoring system which comprises a multicomponent point system based upon abnormalities in serum bilirubin level, serum albumin level, prothrombin time, the presence and severity of ascites, and the presence and severity of encephalopathy. Based upon the presence and severity of abnormality of these parameters, patients may be placed in one of three categories of increasing severity of clinical disease: A, B, or C.
- a therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents is an amount that effects a change of one unit or more in the fibrosis stage based on pre- and post-therapy liver biopsies.
- a therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents reduces liver fibrosis by at least one unit in the METAVIR, the Knodell, the Scheuer, the Ludwig, or the Ishak scoring system.
- indices of liver function can also be used to evaluate the efficacy of treatment with a compound of Formulas XV, XVI, XVII or XVIII. Morphometric computerized semi- automated assessment of the quantitative degree of liver fibrosis based upon specific staining of collagen and/or serum markers of liver fibrosis can also be measured as an indication of the efficacy of a subject treatment method. Secondary indices of liver function include, but are not limited to, serum transaminase levels, prothrombin time, bilirubin, platelet count, portal pressure, albumin level, and assessment of the Child-Pugh score.
- An effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents is an amount that is effective to increase an index of liver function by at least about 10%, at least about 20%, at least about 25%, at least about 30%, at least about 35%, at least about 40%, at least about 45%), at least about 50%>, at least about 55%, at least about 60%>, at least about 65%, at least about 70%>, at least about 75%, or at least about 80%>, or more, compared to the index of liver function in an untreated individual, or to a placebo-treated individual.
- Those skilled in the art can readily measure such indices of liver function, using standard assay methods, many of which are commercially available, and are used routinely in clinical settings.
- Serum markers of liver fibrosis can also be measured as an indication of the efficacy of a subject treatment method.
- Serum markers of liver fibrosis include, but are not limited to, hyaluronate, N-terminal procollagen III peptide, 7S domain of type IV collagen, C-terminal procollagen I peptide, and laminin.
- Additional biochemical markers of liver fibrosis include ⁇ -2-macroglobulin, haptoglobin, gamma globulin, apolipoprotein A, and gamma glutamyl transpeptidase.
- a therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents is an amount that is effective to reduce a serum level of a marker of liver fibrosis by at least about 10%, at least about 20%, at least about 25%, at least about 30%, at least about 35%, at least about 40%), at least about 45%, at least about 50%>, at least about 55%, at least about 60%>, at least about 65%, at least about 70%>, at least about 75%, or at least about 80%, or more, compared to the level of the marker in an untreated individual, or to a placebo-treated individual.
- ELISA enzyme-linked immunosorbent assays
- radioimmunoassays radioimmunoassays
- a "complication associated with cirrhosis of the liver” refers to a disorder that is a sequellae of decompensated liver disease, i.e., or occurs subsequently to and as a result of development of liver fibrosis, and includes, but it not limited to, development of ascites, variceal bleeding, portal hypertension, jaundice, progressive liver insufficiency, encephalopathy, hepatocellular carcinoma, liver failure requiring liver transplantation, and liver-related mortality.
- a therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents is an amount that is effective in reducing the incidence (e.g., the likelihood that an individual will develop) of a disorder associated with cirrhosis of the liver by at least about 10%, at least about 20%), at least about 25%, at least about 30%, at least about 35%, at least about 40%, at least about 45%, at least about 50%, at least about 55%, at least about 60%, at least about 65%, at least about 70%, at least about 75%, or at least about 80%, or more, compared to an untreated individual, or to a placebo-treated individual.
- Reduction in liver fibrosis can increase liver function.
- the embodiments provide methods for increasing liver function, generally involving administering a therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents.
- Liver functions include, but are not limited to, synthesis of proteins such as serum proteins (e.g., albumin, clotting factors, alkaline phosphatase, aminotransferases (e.g., alanine transaminase, aspartate transaminase), 5 '-nucleosidase, ⁇ -glutaminyltranspeptidase, etc.), synthesis of bilirubin, synthesis of cholesterol, and synthesis of bile acids; a liver metabolic function, including, but not limited to, carbohydrate metabolism, amino acid and ammonia metabolism, hormone metabolism, and lipid metabolism; detoxification of exogenous drugs; a hemodynamic function, including splanchnic and portal hemodynamics; and the like.
- proteins such as serum proteins (e.g., albumin, clotting factors, alkaline phosphatase, aminotransferases (e.g., alanine transaminase, aspartate transaminase), 5
- liver function is increased is readily ascertainable by those skilled in the art, using well-established tests of liver function.
- markers of liver function such as albumin, alkaline phosphatase, alanine transaminase, aspartate transaminase, bilirubin, and the like, can be assessed by measuring the level of these markers in the serum, using standard immunological and enzymatic assays.
- Splanchnic circulation and portal hemodynamics can be measured by portal wedge pressure and/or resistance using standard methods.
- Metabolic functions can be measured by measuring the level of ammonia in the serum.
- Whether serum proteins normally secreted by the liver are in the normal range can be determined by measuring the levels of such proteins, using standard immunological and enzymatic assays. Those skilled in the art know the normal ranges for such serum proteins. The following are non-limiting examples.
- the normal level of alanine transaminase is about 45 IU per milliliter of serum.
- the normal range of aspartate transaminase is from about 5 to about 40 units per liter of serum.
- Bilirubin is measured using standard assays. Normal bilirubin levels are usually less than about 1.2 mg/dL.
- Serum albumin levels are measured using standard assays. Normal levels of serum albumin are in the range of from about 35 to about 55 g/L.
- Prolongation of prothrombin time is measured using standard assays. Normal prothrombin time is less than about 4 seconds longer than control.
- a therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents is one that is effective to increase liver function by at least about 10%, at least about 20%, at least about 30%), at least about 40%>, at least about 50%>, at least about 60%>, at least about 70%>, at least about 80%, or more.
- a therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents is an amount effective to reduce an elevated level of a serum marker of liver function by at least about 10%, at least about 20%, at least about 30%, at least about 40%), at least about 50%>, at least about 60%>, at least about 70%>, at least about 80%>, or more, or to reduce the level of the serum marker of liver function to within a normal range.
- a therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents is also an amount effective to increase a reduced level of a serum marker of liver function by at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%), at least about 70%>, at least about 80%>, or more, or to increase the level of the serum marker of liver function to within a normal range.
- the active agent(s) may be administered to the host using any convenient means capable of resulting in the desired therapeutic effect.
- the agent can be incorporated into a variety of formulations for therapeutic administration. More particularly, the agents of the embodiments can be formulated into pharmaceutical compositions by combination with appropriate, pharmaceutically acceptable carriers or diluents, and may be formulated into preparations in solid, semi-solid, liquid or gaseous forms, such as tablets, capsules, powders, granules, ointments, solutions, suppositories, injections, inhalants and aerosols. Other antiviral or antifibrotic agents
- a subject method will in some embodiments be carried out by administering a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agent(s).
- the method further includes administration of one or more interferon receptor agonist(s)
- the method further includes administration of pirfenidone or a pirfenidone analog.
- Additional antiviral agents that are suitable for use in combination therapy include, but are not limited to, nucleotide and nucleoside analogs.
- Non-limiting examples include azidothymidine (AZT) (zidovudine), and analogs and derivatives thereof; 2',3 '-dideoxyinosine (DDI) (didanosine), and analogs and derivatives thereof; 2',3 '-dideoxycytidine (DDC) (dideoxycytidine), and analogs and derivatives thereof; 2',3 '-didehydro-2',3 '-dideoxythymidine (D4T) (stavudine), and analogs and derivatives thereof; combivir; abacavir; adefovir dipoxil; cidofovir; ribavirin; ribavirin analogs; and the like.
- the method further includes administration of ribavirin.
- Ribavirin, l- -D-ribofuranosyl-lH-l ,2,4-triazole-3-carboxamide available from ICN Pharmaceuticals, Inc., Costa Mesa, Calif, is described in the Merck Index, compound No. 8199, Eleventh Edition. Its manufacture and formulation is described in U.S. Pat. No. 4,21 1 ,771. Some embodiments also involve use of derivatives of ribavirin (see, e.g. , U.S. Pat. No. 6,277,830).
- the ribavirin may be administered orally in capsule or tablet form, or in the same or different administration form and in the same or different route as the subject compound.
- other types of administration of both medicaments as they become available are contemplated, such as by nasal spray, transdermally, intravenously, by suppository, by sustained release dosage form, etc. Any form of administration will work so long as the proper dosages are delivered without destroying the active ingredient.
- the method further includes administration of ritonavir.
- Ritonavir 10-hydroxy-2-methyl-5 -( 1 -methy lethyl)- 1 - [2-( 1 -methylethyl)-4- thiazolyl]-3,6-dioxo-8,l l-bis(phenylmethyl)-2,4,7,12-tetraazatridecan-13-oic acid, 5- thiazolylmethyl ester [5i -(5i?*,8i?*,10i?*,l li?*)], available from Abbott Laboratories, is an inhibitor of the protease of the human immunodeficiency virus and also of the cytochrome P450 3 A and P450 2D6 liver enzymes frequently involved in hepatic metabolism of therapeutic molecules in man.
- the method further includes administration of a protease inhibitor. In some embodiments, the method further includes administration of an NS5A inhibitor. In some embodiments, the method further includes administration of a helicase inhibitor. In some embodiments, the method further includes administration of a polymerase inhibitor.
- an additional antiviral agent is administered during the entire course of the subject compound treatment.
- an additional antiviral agent is administered for a period of time that is overlapping with that of the subject compound treatment, e.g., the additional antiviral agent treatment can begin before the subject compound treatment begins and end before the subject compound treatment ends; the additional antiviral agent treatment can begin after the subject compound treatment begins and end after the subject compound treatment ends; the additional antiviral agent treatment can begin after the subject compound treatment begins and end before the subject compound treatment ends; or the additional antiviral agent treatment can begin before the subject compound treatment begins and end after the subject compound treatment ends.
- the compounds as described herein may be used in acute or chronic therapy for HCV disease.
- the compounds as described herein can be administered for a period of about 1 day to about 7 days, or about 1 week to about 2 weeks, or about 2 weeks to about 3 weeks, or about 3 weeks to about 4 weeks, or about 1 month to about 2 months, or about 3 months to about 4 months, or about 4 months to about 6 months, or about 6 months to about 8 months, or about 8 months to about 12 months, or at least one year, and may be administered over longer periods of time.
- the compounds as described herein can be administered 5 times per day, 4 times per day, tid, bid, qd, qod, biw, tiw, qw, qow, three times per month, or once monthly.
- the NS5A inhibitor compound is administered as a continuous infusion.
- an compounds as described herein of the embodiments can be administered orally.
- a compound as described herein may be administered to the patient at a dosage from about 0.01 mg to about 100 mg/kg patient bodyweight per day, in 1 to 5 divided doses per day.
- the compounds as described herein can be administered at a dosage of about 0.5 mg to about 75 mg/kg patient bodyweight per day, in 1 to 5 divided doses per day.
- the amount of active ingredient that may be combined with carrier materials to produce a dosage form can vary depending on the host to be treated and the particular mode of administration.
- a typical pharmaceutical preparation can contain from about 5% to about 95% active ingredient (w/w). In other embodiments, the pharmaceutical preparation can contain from about 20% to about 80% active ingredient.
- dose levels can vary as a function of the specific compound, the severity of the symptoms and the susceptibility of the subject to side effects.
- Preferred dosages for a given compound as described herein can be readily determinable by those of skill in the art by a variety of means.
- a preferred means can be to measure the physiological potency of a given interferon receptor agonist.
- multiple doses of NS5A inhibitor compound are administered.
- an NS5A inhibitor compound is administered once per month, twice per month, three times per month, every other week (qow), once per week (qw), twice per week (biw), three times per week (tiw), four times per week, five times per week, six times per week, every other day (qod), daily (qd), twice a day (qid), or three times a day (tid), over a period of time ranging from about one day to about one week, from about two weeks to about four weeks, from about one month to about two months, from about two months to about four months, from about four months to about six months, from about six months to about eight months, from about eight months to about 1 year, from about 1 year to about 2 years, or from about 2 years to about 4 years, or more.
- Some embodiments provide a method of treating an HCV infection in an individual having an HCV infection, the method comprising administering an effective amount of one of the compounds as decribed herein, and effective amount of a TNF-a antagonist, and an effective amount of one or more interferons.
- the specific regimen of drug therapy used in treatment of the HCV patient is selected according to certain disease parameters exhibited by the patient, such as the initial viral load, genotype of the HCV infection in the patient, liver histology and/or stage of liver fibrosis in the patient.
- Any of the above treatment regimens can be administered to individuals who have been diagnosed with an HCV infection. Any of the above treatment regimens can be administered to individuals having advanced or severe stage liver fibrosis as measured by a Knodell score of 3 or 4 or no or early stage liver fibrosis as measured by a Knodell score of 0, 1, or 2. Any of the above treatment regimens can be administered to individuals who have failed previous treatment for HCV infection ("treatment failure patients," including non-responders and relapsers).
- Individuals who have been clinically diagnosed as infected with HCV are of particular interest in many embodiments.
- Individuals who are infected with HCV are identified as having HCV R A in their blood, and/or having anti-HCV antibody in their serum.
- Such individuals include anti-HCV ELISA-positive individuals, and individuals with a positive recombinant immunoblot assay (RIB A).
- RIB A positive recombinant immunoblot assay
- Such individuals may also, but need not, have elevated serum ALT levels.
- na ' ive individuals e.g., individuals not previously treated for HCV, particularly those who have not previously received IFN-a-based and/or ribavirin-based therapy
- individuals who have failed prior treatment for HCV (“treatment failure" patients).
- Treatment failure patients include non-responders (i.e., individuals in whom the HCV titer was not significantly or sufficiently reduced by a previous treatment for HCV, e.g., a previous IFN-a monotherapy, a previous IFN-a and ribavirin combination therapy, or a previous pegylated IFN-a and ribavirin combination therapy); and relapsers (i.e., individuals who were previously treated for HCV, e.g., who received a previous IFN-a monotherapy, a previous IFN-a and ribavirin combination therapy, or a previous pegylated IFN-a and ribavirin combination therapy, whose HCV titer decreased, and subsequently increased).
- non-responders i.e., individuals in whom the HCV titer was not significantly or sufficiently reduced by a previous treatment for HCV, e.g., a previous IFN-a monotherapy, a previous IFN-a and ribavirin combination therapy,
- individuals have an HCV titer of at least about 10 5 , at least about 5 x 10 5 , or at least about 10 6 , or at least about 2 x 10 6 , genome copies of HCV per milliliter of serum.
- the patient may be infected with any HCV genotype (genotype 1, including la and lb, 2, 3, 4, 6, etc. and subtypes (e.g., 2a, 2b, 3a, etc.)), particularly a difficult to treat genotype such as HCV genotype 1 and particular HCV subtypes and quasispecies.
- HCV -positive individuals (as described above) who exhibit severe fibrosis or early cirrhosis (non-decompensated, Child' s-Pugh class A or less), or more advanced cirrhosis (decompensated, Child' s-Pugh class B or C) due to chronic HCV infection and who are viremic despite prior anti-viral treatment with IFN-a- based therapies or who cannot tolerate IFN-a-based therapies, or who have a contraindication to such therapies.
- HCV-positive individuals with stage 3 or 4 liver fibrosis according to the METAVIR scoring system are suitable for treatment with the methods described herein.
- individuals suitable for treatment with the methods of the embodiments are patients with decompensated cirrhosis with clinical manifestations, including patients with far- advanced liver cirrhosis, including those awaiting liver transplantation.
- individuals suitable for treatment with the methods described herein include patients with milder degrees of fibrosis including those with early fibrosis (stages 1 and 2 in the METAVIR, Ludwig, and Scheuer scoring systems; or stages 1, 2, or 3 in the Ishak scoring system.).
- Example XI-I General synthetic protocol for the preparation of compounds 701-705 -I
- N-Boc-L-phenylalanine 200 mg, 0.75 mmol
- BOP 66 mg, 1.5 mmol
- DIEA 290 mg, 2.3 mmol
- CD 3 OD ⁇ 7.60-7.86 (m, 8 H), 7.20-7.25 (m, 2H), 5.14-5.20 (m, 1H), 4.14-4.20 (m, 2 H), 3.81-4.09 (m, 4 H), 3.62(s, 6 H), 2.49-2.51 (m, 1 H), 2.09-2.35 (m, 5 H), 1.94-2.04 (m, 4 H), 1.83 (s, 3 H), 0.83-0.96 (m, 12 H).
- Example IX-IV Compound 901 can be prepared according to the following Scheme:
- Example IX- VII Compound 904 can be prepared according to the following Scheme:
- Example IX- VIII Compound 905 can be prepared according to the following Scheme:
- Example IX-X Compound 907 can be prepared according to the following Scheme:
- Example IX-XI Compound 908 can be prepared according to the following Scheme:
- Example IX-XII Compound 909 can be prepared according to the following Scheme:
- Example XV-I Compound 1001 can be prepared according to the following Schemes:
- Example XV-II Compound 1002 can be prepared according to the following Scheme:
- Example XV-III Compound 1003 can be prepared according to the following Schemes:
- Example XV-IV Compound 1004 can be prepared according to the following Schemes:
- Example XV- V Compound 1005 can be prepared according to the following Schemes:
- Example XV- VI Compound 1006 can be prepared according to the following Scheme:
- Example XV- VII Compound 1007 can be prepared according to the following Schemes:
- Example XV- VIII Compound 1008 can be prepared according to the following Schemes:
Abstract
The embodiments provide compounds of the general Formulae XV, XVI, XVII and XVIII as well as compositions, including pharmaceutical compositions, comprising a subject compound. The embodiments further provide treatment methods, including methods of treating a hepatitis C virus infection and methods of treating liver fibrosis, the methods generally involving administering to an individual in need thereof an effective amount of a subject compound or composition.
Description
NOVEL INHIBITORS OF HEPATITIS C VIRUS REPLICATION
RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Provisional Application Nos. 61/425,718, filed December 21, 2010; and 61/ 454,438, filed March 18, 2011; both of which are incorporated herein by reference in their entirety.
BACKGROUND
Field of the Invention
[0002] The embodiments described herein relate to compounds, processes for their synthesis, compositions and methods for the therapeutic use of the compounds, such as for treating hepatitis C virus (HCV) infection.
Description of the Related Art
[0003] Hepatitis C virus (HCV) infection is the most common chronic blood borne infection in the United States. Although the numbers of new infections have declined, the burden of chronic infection is substantial, with Centers for Disease Control estimates of 3.9 million (1.8%) infected persons in the United States. Chronic liver disease is the tenth leading cause of death among adults in the United States, and accounts for approximately 25,000 deaths annually, or approximately 1% of all deaths. Studies indicate that 40% of chronic liver disease is HCV-related, resulting in an estimated 8,000-10,000 deaths each year. HCV-associated end-stage liver disease is the most frequent indication for liver transplantation among adults.
[0004] Antiviral therapy of chronic hepatitis C has evolved rapidly over the last decade, with significant improvements seen in the efficacy of treatment. Nevertheless, even with combination therapy using pegylated IFN-a plus ribavirin, 40%> to 50% of patients fail therapy; they are nonresponders or relapsers. These patients currently have no effective therapeutic alternative. In particular, patients who have advanced fibrosis or cirrhosis on liver biopsy are at significant risk of developing complications of advanced liver disease, including ascites, jaundice, variceal bleeding, encephalopathy, and progressive liver failure, as well as a markedly increased risk of hepatocellular carcinoma.
[0005] The high prevalence of chronic HCV infection has important public health implications for the future burden of chronic liver disease in the United States.
Data derived from the National Health and Nutrition Examination Survey (NHANES III) indicate that a large increase in the rate of new HCV infections occurred from the late 1960s to the early 1980s, particularly among persons between 20 to 40 years of age. It is estimated that the number of persons with long-standing HCV infection of 20 years or longer could more than quadruple from 1990 to 2015, from 750,000 to over 3 million. The proportional increase in persons infected for 30 or 40 years would be even greater. Since the risk of HCV-related chronic liver disease is related to the duration of infection, with the risk of cirrhosis progressively increasing for persons infected for longer than 20 years, this will result in a substantial increase in cirrhosis-related morbidity and mortality among patients infected between the years of 1965-1985.
[0006] HCV is an enveloped positive strand RNA virus in the Flaviviridae family. The single strand HCV RNA genome is believed to be approximately 9500 nucleotides in length and has a single open reading frame (ORF) encoding a single large polyprotein of about 3000 amino acids. In infected cells, it is believed that this polyprotein is cleaved at multiple sites by cellular and viral proteases to produce the structural and non-structural (NS) proteins of the virus. In the case of HCV, the generation of mature nonstructural proteins (NS2, NS3, NS4, NS4A, NS4B, NS5A, and NS5B) is believed to be effected by two viral proteases. The first viral protease is believed to cleave at the NS2-NS3 junction of the polyprotein. The second viral protease is believed to be a serine protease contained within the N-terminal region of NS3 (herein referred to as "NS3 protease"). NS3 protease is believed to mediate all of the subsequent cleavage events at sites downstream relative to the position of NS3 in the polyprotein (i.e., sites located between the C-terminus of NS3 and the C-terminus of the polyprotein). NS3 protease exhibits activity both in cis, at the NS3-NS4 cleavage site, and in trans, for the remaining NS4A-NS4B, NS4B-NS5A, and NS5A-NS5B sites. The NS4A protein is believed to serve multiple functions, acting as a cofactor for the NS3 protease and possibly assisting in the membrane localization of NS3 and other viral replicase components. Apparently, the formation of the complex between NS3 and NS4A may be necessary for NS3-mediated processing events and enhances proteolytic efficiency at all sites recognized by NS3. The NS3 protease also appears to exhibit nucleoside triphosphatase and RNA helicase activities. NS5B is believed to be an RNA-dependent RNA polymerase involved in the replication of HCV RNA. In addition, compounds that
inhibit the action of NS5 A in viral replication are potentially useful for the treatment of HCV.
SUMMARY
[0007] Some embodiments provide a compound having the structure of Formula XV:
□ 12 13
I
L6— L4
L7-Q7
XV
or a pharmaceutically acceptable salt thereof,
wherein:
12 13 12 13
each R R TSi is separately selected, wherein R and R are each separately selected from hydrogen, -[(Y14)(C(R2)2)r( R2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3-7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab. or R12R13N is a heterocyclyl linked through a ring nitrogen atom optionally substituted with one or more of oxo, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-
Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab.
each Rlab is separately selected from the group consisting of -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80,
arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)n-, (RcRdN)(CH=CH)m- (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R80 is separately selected from the group consisting of hydrogen, alkoxyalkyl, Ci_6alkyl, C3-7cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and (ReRfN)alkyl, said alkoxyalkyl, Ci_6alkyl, C3_7cycloalkyl, aryl, arylalkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (ReRfN)alkyl are each optionally substituted with one or more Rlac;
each Rlac is separately selected from the group consisting of -C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-,
Ci_6alkylC(=0)Ci_6alkyl, aryl, aryl(CH2)„-, aryl(CH2)nO-, aryl(CH=CH)m-, arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)„-, (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each Y14 is separately selected from the group consisting of -C(=0)-, - S(=0)-, -C(=S)-, -S(=0)2-, -C(=0)0-, -C(=0)NR2\ -S(=0)2NR2\ - C(=0)NR2cC(=0)-, and -C(CF3)2NR2c-,
Y2 is selected from O (oxygen), S (sulfur), S(O), S02, NR2, and C(R2)2 with the proviso that when X2 is null Y2 is C(R2)2;
each X10 is (C(R2)2)q;
each X11 is separately selected from the group consisting of (C(R2)2)q, and
each Y11 is separately selected from the group consisting of -0(C(R2)2)n-, -S(C(R2)2)„-, -S(0)(C(R2)2)n-, -S02(C(R2)2)n-, -NR2(C(R2)2)„-, and (C(R2)2)q;
R1 is selected from the group consisting of Rlaa, RlaC(=0)- and RlaC(=S)-;
each Rla is separately selected from the group consisting of -C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-, Ci_6alkylOC(=0)Ci_6alkyl, Ci_6alkylC(=0)Ci_6alkyl, aryl, aryl(CH2)„-, aryl(CH2)nO-, aryl(CH=CH)m- arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)„-, (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
Rlaa is -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s- (Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab.
each R10 is RcRdN-;
each R is separately selected from the group consisting of H (hydrogen), alkoxyalkyl, Ci_6alkylOC(=0)Ci_6alkyl, Ci_6alkylC(=0)Ci_6alkyl, aryl(CH2)„- arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN(CH2)n-, (RcRdN)alkyl, and Ci_6alkyl optionally substituted with up to 9 halo;
each RcRdN is separately selected, wherein Rc and Rd are each separately selected from hydrogen, alkoxyC(=0)-, Ci_6alkyl, Ci_6alkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-, wherein the alkyl part of arylalkyl, arylalkylC(=0)-, heterocyclylalkyl, and heterocyclylalkylC(=0)- are each optionally substituted with one ReRfN- group; and wherein the aryl part of arylalkyl, arylalkylC(=0)-, arylC(=0)-, and arylsulfonyl, and the heterocyclyl part of heterocyclylalkyl, heterocyclylalkylC(=0)-, and heterocyclylC(=0)- are each optionally substituted with up to three substituents each independently selected from the group consisting of cyano, halo, nitro, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each ReRfN is separately selected, wherein Re and Rf are each separately selected from hydrogen, Ci_6alkyl, aryl, arylalkyl, cycloalkyl, (cyclolalkyl)alkyl, heterocyclyl, heterocyclylalkyl, (RxRyN)alkyl, and (RxRyN)C(=0)-;
each RxRyN is separately selected, wherein Rx and Ry are each separately selected from hydrogen, Ci_6alkylOC(=0)-, Ci_6alkyl, Ci_6alkylC(=0)-, aryl, arylalkyl, cycloalkyl, and heterocyclyl;
each C(R2a)2 is separately selected, wherein each R2a is separately selected from the group consisting of hydrogen, Ci_6alkyl optionally substituted with up to 9 halo, aryl(CH2)n-, and heteroaryl(CH2)n-, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo and Ci_6alkyl optionally substituted with up to 9
halo, or optionally C(R2a)2 is
each R3a is separately selected from the group consisting of hydrogen, and optionally substituted Ci_6alkyl;
each R3b is separately selected from the group consisting of optionally substituted Ci_6alkyl, heteroaryl, -(CH2)nC(=0)NR4aR4b, -(CH2)nC(=0)OR5a, and -(CH2)nC(=0)R6a said heteroaryl optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R4aR4bN is separately selected, wherein R4a and R4b are each separately selected from the group consisting of hydrogen, optionally substituted Ci_6alkyl, and aryl(CH2)„-;
each R5a is separately selected from the group consisting of optionally substituted Ci_6alkyl, and aryl(CH2)„-;
each R6a is separately selected from the group consisting of optionally substituted Ci_6alkyl, and aryl(CH2)„-;
each A1 is separately selected from the group consisting of C2_6alkenyl, Ci_6alkyl, and -(CH2)n-0-(CH2)m-, each optionally substituted with one or more
R2;
each R2 is separately selected, wherein R2 is selected from the group consisting of hydrogen, halo, hydroxy, Ci_6alkoxy, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, alkyoxyalkyl, C3_7Cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, heterocyclylalkyl, (ReRfN)alkyl, and RaRbN-, said Ci_6alkyl optionally substituted with one or more halo, -OR2b, -C(=0)OR2b, - C(=0)NHR2b, -NHC (=NH)NHR2b , -NHR2b, SR2b, imidazolyl, indolyl, -SCH3, phenyl, and 4-hydroxyphenyl, said Ci_6alkoxy, alkoxyalkyl, aryl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, arylalkyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (ReRfN)alkyl each optionally substituted with one or more R4, or optionally two vicinal R2 and the carbons to which they are attached are together a fused three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups;
R2b is selected from the group consisting of hydrogen, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl;
each R c is selected from the group consisting of hydrogen, Ci_6alkyl, C2_6alkenyl, C2-6alkynyl, C3-7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl, said alkyl optionally substituted with ReRfN-, alkoxy, or Ci_6alkylS-;
each RaRbN is separately selected, wherein Ra and Rb are each separately selected from the group consisting of hydrogen, C2_6alkenyl, and Ci_6alkyl;
OC(R2)2-, ¾ H f -C(CF3)2NR2c-, NH, and -(CH=CH)-;
J2 is aryl, heteroaryl, heterocyclyl, cycloalkyl, cycloalkenyl, or polycyclic hydrocarbon, each optionally substituted with one or more R15;
each R14 is separately selected from the group consisting of hydroxy, Ci_6alkoxy optionally substituted with up to 9 fluoro, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R15 is separately selected from the group consisting of halo, hydroxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, R9, RxRyN, RxRyNC(=0), RxRyNCi_6alkyl, heteroaryl, aryl, Ci_6alkyl optionally substituted with up to 9 halo, Ci_6alkyl substituted with up to 5 hydroxy, Ci_6alkoxy optionally substituted with up to 9 halo, Ci_6haloalkyl, RaRbN-, (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl substituted with up to 5 hydroxy, said substituent aryl and heteroaryl are each optionally substituted with one or more R14, or optionally two vicinal R15 and the carbons to which they are attached are together a fused three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups, or optionally two geminal R15 and the carbon to which they are attached are together a three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups, or optionally two geminal R15 are together oxo;
each L5 is separately selected from the group consisting of
C(R2)2, -C(R2)20-, -C(=0)-, O (oxygen), NH, and -(CH=CH)-;
each A is separately selected from the group consisting of CR3 and N (nitrogen);
each R3 is separately selected from the group consisting of hydrogen, Ci_6alkoxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, hydroxy, RaRbN- (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy;
L6 is selected from the group consisting of
7 is selected from the group consisting
each X is separately selected from the group consisting of CR and N (nitrogen);
each Y4 is separately selected from the group consisting of C(R4)2, NR4, O (oxygen), and S (sulfur);
each X9 is separately selected from the group consisting of CH and N (nitrogen);
each Y10 is separately selected from the group consisting of -CH2- and -
NH-;
each m separately is 1 or 2;
each n separately is 0, 1 or 2;
each p separately is 1, 2, 3 or 4;
each q separately is 1, 2, 3, 4 or 5;
each r separately is 0, 1, 2, 3, or 4;
each s separately is 0 or 1 ;
each R4 is separately selected from the group consisting of H (hydrogen), Ci_6alkoxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, Ci_6haloalkyl, hydroxy, RaRbN-, (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy, or optionally two geminal R4 are together oxo;
R9 is selected from the group consisting of hydrogen and -C(=0)R9a; R9a is selected from the group consisting of -NR9bR9c, -OR9d, Ci_ Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
R9b is selected from the group consisting of hydrogen, Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
R9c is selected from the group consisting of Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl; and
R9d is selected from the group consisting of Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl.
[0008] In some embodiments of Formula XV, at least one is a nitrogen containing heterocyclyl optionally including one or more -C(=0)-, O (oxygen), S (sulfur), S(O), S02, or NH groups in the heterocyclyl ring and optionally substituted with one or more of -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s- (Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl,
arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more R1.
[0009] In some embodiments of Formula XV, at least one is
optionally substituted with one or more of -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3-7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more R1; wherein Y3 is O (oxygen), S (sulfur), S(O), S02, -C(=0)-, -NH-, -CH2-, -NHCH2-, -OCH2-, -SCH2-, -NHCH2-, -NHC(=0)-, or -NHS02-.
[0010] In some embodiments, the compound of Formula XV has the structure of Formula XV
, or a pharmaceutically acceptable salt thereof.
[0011] In some embodiments, the compound of Formula XV has the structure:
-13-
or a pharmaceutically acceptable salt thereof.
[0012] In some embodiments of Formula XV, L4 can be selected from the
each B can separately be selected, wherein B can be a fused optionally substituted saturated or unsaturated three- to seven-membered carbocyclic ring or
a fused optionally substituted saturated or unsaturated three- to seven-membered heterocyclic ring, each optionally substituted with one or more R4;
L2 can be selected from the group consisting of -C(=0)-, -(CH2CH2)-, -(CH20)-, -(CH2S)-, -(CH=CH)-, -(CH=N)-, -NH-, O (oxygen), S (sulfur), and -CH2-;
o
L3 can be selected from the group consisting of H , -(NR9)-, O (oxygen), S (sulfur), and -CH2-;
X3 can be selected from the group consisting of NH, O (oxygen), and S (sulfur);
each Xs can be separately selected from the group consisting of -NH-, O (oxygen), S (sulfur), and -CH2-;
each X6 can be separately selected from the group consisting of N (nitrogen), and CR8; and
each R8 can be separately selected from the group consisting of hydrogen, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, halo, (RaRbN)alkyl, (RaRbN)C(=0)-, and Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy.
[0013] In some embodiments of Formula XV, L4 can be selected from the
R6 can be Ci_6alkyl optionally substituted with up to 9 halo.
[0015] In some embodiments of Formula XV, R6 can be methyl.
[0016] Some embodiments provide a compound having the structure of Formula XVI:
XVI
or a pharmaceutically acceptable salt thereof,
wherein:
R 1 2 F ,13 lected from the group consisting of ■n~" , and
each Y2 is selected from O (oxygen), S (sulfur), S(O), S02, NR2, and C(R2)2 with the proviso that when X2 is null Y2 is C(R2)2;
each X10 is (C(R2)2)q;
each Y11 is separately selected from the group consisting of -0(C(R2)2)n-, -S(C(R2)2)n-, -S(0)(C(R2)2)n-, -S02(C(R2)2)n-, -NR2(C(R2)2)n-, and (C(R2)2)q;
12 13 12 13
each R R TSi is separately selected, wherein R and R are each separately selected from hydrogen, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-,
arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3-7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab.
or R12R13N is a heterocyclyl linked through a ring nitrogen atom optionally substituted with one or more of oxo, -[(Y14)(C(R2)2)r( R2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab.
each Rlab is separately selected from the group consisting of -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80,
-C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-,
Ci_6alkylC(=0)Ci_6alkyl, aryl, aryl(CH2)„-, aryl(CH2)nO-, aryl(CH=CH)m-, arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)„-, (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R80 is separately selected from the group consisting of hydrogen, alkoxyalkyl, Ci_6alkyl, C3_7cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and (ReRfN)alkyl, said alkoxyalkyl, Ci_6alkyl, C3_7cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (ReRfN)alkyl are each optionally substituted with one or more Rlac;
each Rlac is separately selected from the group consisting of -C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-,
Ci_6alkylC(=0)Ci_6alkyl, aryl, aryl(CH2)„-, aryl(CH2)nO-, aryl(CH=CH)m- arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)„-, (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each Y14 is separately selected from the group consisting of -C(=0)-, - S(=0)-, -C(=S)-, -S(=0)2-, -C(=0)0-, -C(=0)NR2\ -S(=0)2NR2\ - C(=0)NR2cC(=0)-, and -C(CF3)2NR2c-,
R1 is selected from the group consisting of Rlaa, RlaC(=0)- and RlaC(=S)-;
each Rla is separately selected from the group consisting of -C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-, Ci_6alkylOC(=0)Ci_6alkyl, Ci_6alkylC(=0)Ci_6alkyl, aryl, aryl(CH2)„-, aryl(CH2)nO-, aryl(CH=CH)m-, arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)„-, (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
Rlaa is selected from the group consisting of -C(R2a)2NR3aR3b, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3-7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab. each R10 is RcRdN-;
each R11 is separately selected from the group consisting of H (hydrogen), alkoxyalkyl, Ci_6alkylOC(=0)Ci_6alkyl, Ci_6alkylC(=0)Ci_6alkyl, aryl(CH2)„-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)alkyl, cycloalkylOalkyl,
heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN(CH2)n-, (RcRdN)alkyl, and Ci_6alkyl optionally substituted with up to 9 halo;
each RcRdN is separately selected, wherein Rc and Rd are each separately selected from hydrogen, alkoxyC(=0)-, Ci_6alkyl, Ci_6alkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-, wherein the alkyl part of arylalkyl, arylalkylC(=0)-, heterocyclylalkyl, and heterocyclylalkylC(=0)- are each optionally substituted with one ReRfN- group; and wherein the aryl part of arylalkyl, arylalkylC(=0)-, arylC(=0)-, and arylsulfonyl, and the heterocyclyl part of heterocyclylalkyl, heterocyclylalkylC(=0)-, and heterocyclylC(=0)- are each optionally substituted with up to three substituents each independently selected from the group consisting of cyano, halo, nitro, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each ReRfN is separately selected, wherein Re and Rf are each separately selected from hydrogen, Ci_6alkyl, aryl, arylalkyl, cycloalkyl, (cyclolalkyl)alkyl, heterocyclyl, heterocyclylalkyl, (RxRyN)alkyl, and (RxRyN)C(=0)-;
each RxRyN is separately selected, wherein Rx and Ry are each separately selected from hydrogen, Ci_6alkylOC(=0)-, Ci_6alkyl, Ci_6alkylC(=0)-, aryl, arylalkyl, cycloalkyl, and heterocyclyl;
each C(R2a)2 is separately selected, wherein each R2a is separately selected from the group consisting of hydrogen, Ci_6alkyl optionally substituted with up to 9 halo, aryl(CH2)„-, and heteroaryl(CH2)„-, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo and Ci_6alkyl optionally substituted with up to 9
each R3a is separately selected from the group consisting of hydrogen, and optionally substituted Ci_6alkyl;
each R3b is separately selected from the group consisting of optionally substituted Ci_6alkyl, heteroaryl, -(CH2)nC(=0)NR4aR4b, -(CH2)nC(=0)OR5a,
and -(CH2)nC(=0)R a said heteroaryl optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R4aR4bN is separately selected, wherein R4a and R4b are each separately selected from the group consisting of hydrogen, optionally substituted Ci_6alkyl, and aryl(CH2)n-;
each R5a is separately selected from the group consisting of optionally substituted Ci_6alkyl, and aryl(CH2)„-;
each R6a is separately selected from the group consisting of optionally substituted Ci_6alkyl, and aryl(CH2)n-;
each A1 is separately selected from the group consisting of C2_6alkenyl, Ci_6alkyl, and -(CH2)n-0-(CH2)m-, each optionally substituted with one or more
R2;
each R2 is separately selected, wherein R2 is selected from the group consisting of hydrogen, halo, hydroxy, Ci_6alkoxy, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, alkyoxyalkyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, heterocyclylalkyl, (ReRfN)alkyl, RaRbN-, said Ci_6alkyl optionally substituted with one or more halo, -OR2b, -C(=0)OR2b, - C(=0)NHR2b, -NHC (=NH)NHR2b , -NHR2b, SR2b, imidazolyl, indolyl, -SCH3, phenyl, and 4-hydroxyphenyl, said Ci_6alkoxy, alkoxyalkyl, aryl, C2_6alkenyl, C2_6alkynyl, C3_7Cycloalkyl, arylalkyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (ReRfN)alkyl each optionally substituted with one or more R4, or optionally two vicinal R2 and the carbons to which they are attached are together a fused three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups;
R2b is selected from the group consisting of hydrogen, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl;
each R2c is selected from the group consisting of hydrogen, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl, said alkyl optionally substituted with ReRfN-, alkoxy, or Ci_6alkylS-;
each R^^ is separately selected, wherein Ra and Rb are each separately selected from the group consisting of hydrogen, C2_6alkenyl, and Ci_6alkyl;
L4 is selected from the group consisting of -(J2)S-(L5)S-(J2)S-(L5)S-J2-
, f -C(=0)-, O (oxygen), - o
OC(R2)2-, ¾ H , -C(CF3)2NR2c-, NH, and -(CH=CH)-;
J2 is aryl, heteroaryl, heterocyclyl, cycloalkyl, cycloalkenyl, or polycyclic hydrocarbon, each optionally substituted with one or more R15;
each R14 is separately selected from the group consisting of hydroxy, Ci_6alkoxy optionally substituted with up to 9 fluoro, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R15 is separately selected from the group consisting of halo, hydroxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, R9, RxRyN, RxRyNC(=0), RxRyNCi_6alkyl, heteroaryl, aryl, Ci_6alkyl optionally substituted with up to 9 halo, Ci_6alkyl substituted with up to 5 hydroxy, Ci_6alkoxy optionally substituted with up to 9 halo, Ci_6haloalkyl, RaRbN-, (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl substituted with up to 5 hydroxy, said substituent aryl and heteroaryl are each optionally substituted with one or more R14, or optionally two vicinal R15 and the carbons to which they are attached are together a fused three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups, or optionally two geminal R15 and the carbon to which they are attached are together a three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups, or optionally two geminal R15 are together oxo;
C(R2)2, -C(R2)20-, -C(=0)-, O (oxygen), NH, and -(CH=CH)-
each A is separately selected from the group consisting of CR and N (nitrogen);
each R3 is separately selected from the group consisting of hydrogen, Ci_6alkoxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, hydroxy, RaRbN- (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy;
L6 is selected from the group consisting of
L is selected from the group consisting of
each X is separately selected from the group consisting of CR4 and N (nitrogen);
each Y4 is separately selected from the group consisting of C(R4)2, NR4, O (oxygen), and S (sulfur);
each X9 is separately selected from the group consisting of CH and N (nitrogen);
each Y10 is separately selected from the group consisting of -CH2- and -
NH-;
each m separately is 1 or 2;
each n separately is 0, 1 or 2;
each p separately is 1, 2, 3 or 4;
each q separately is 1, 2, 3, 4 or 5;
each r separately is 0, 1, 2, 3, or 4;
each s separately is 0 or 1 ;
each R4 is separately selected from the group consisting of H (hydrogen), Ci_6alkoxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, Ci_6haloalkyl, hydroxy, RaRbN-, (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy, or optionally two geminal R4 are together oxo;
R9 is selected from the group consisting of hydrogen and -C(=0)R9a;
R9a is selected from the group consisting of -NR9bR9c, -OR9d, Ci_ Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
R9b is selected from the group consisting of hydrogen, Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
R9c is selected from the group consisting of Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl; and
R9d is selected from the group consisting of Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl.
[0017] In some embodiments, the compound of Formula XVI has the structure:
[0018] In some embodiments of Formula XVI, R6 can be methyl.
[0019] In some embodiments, the compound of Formula XVI has the structure of Formula XVIa:
XVIa
or a pharmaceutically acceptable salt thereof, wherein R6 can be Ci_6alkyl optionally substituted with up to 9 halo.
[0020] In some embodiments of Formula XVIa, L4 can be selected from the group consisting of
o
L3 can be selected from the group consisting of H , -(NR9)-, O (oxygen), S (sulfur), and -CH2-;
L can be selected from the group consisting of
, , 0 (oxygen), NH, and -(CH=CH)-;
each X3 can separately be selected from the group consisting of NH, O (oxygen), and S (sulfur);
each Xs can separately be selected from the group consisting of -NH-, O (oxygen), S (sulfur), and -CH2-;
each X6 can separately be selected from the group consisting of N (nitrogen), and CR8; and
each B can separately be selected, wherein B can be a fused optionally substituted saturated or unsaturated three- to seven-membered carbocyclic ring or a fused optionally substituted saturated or unsaturated three- to seven-membered heterocyclic ring, each optionally substituted with one or more R4
[0021] Some embodiments provide a compound having the structure of Formula XVII:
XVII
or a pharmaceutically acceptable salt thereof,
wherein:
□ 12 p 13
R VR
Q7 is selected from the group consisting of ■n~v , and
each X2 is (C(R2)2)q, , or X is null;
each Y2 is selected from O (oxygen), S (sulfur), S(O), S02, NR2, and C(R2)2 with the proviso that when X2 is null Y2 is C(R2)2;
each X10 is (C(R2)2)q;
each Y11 is separately selected from the group consisting of -0(C(R2)2)n-,
-S(C(R2)2)n-, -S(0)(C(R2)2)n-, -S02(C(R2)2)n-, -NR2(C(R2)2)n-, and (C(R2)2)q;
12 13 12 13
each R R TSi is separately selected, wherein R and R are each separately selected from hydrogen, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3-7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab. or R12R13N is a heterocyclyl linked through a ring nitrogen atom optionally substituted with one or more of oxo, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-,
arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3-7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab.
each Rlab is separately selected from the group consisting of -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80,
-C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-, Ci_6alkylOC(=0)Ci_6alkyl, Ci_6alkylC(=0)Ci_6alkyl, aryl, aryl(CH2)„-, aryl(CH2)nO-, aryl(CH=CH)m-, arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)„-, (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R80 is separately selected from the group consisting of hydrogen, alkoxyalkyl, Ci_6alkyl, C3-7cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and (ReRfN)alkyl, said alkoxyalkyl, Ci_6alkyl, C3_7cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (ReRfN)alkyl are each optionally substituted with one or more Rlac;
each Rlac is separately selected from the group consisting of -C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-, Ci_6alkylOC(=0)Ci_6alkyl,
arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)n-, (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each Y14 is separately selected from the group consisting of -C(=0)-, - S(=0)-, -C(=S)-, -S(=0)2-, -C(=0)0-, -C(=0)NR2\ -S(=0)2NR2\ - C(=0)NR2cC(=0)-, and -C(CF3)2NR2c-,
R1 is selected from the group consisting of Rlaa, RlaC(=0)- and RlaC(=S)-;
each Rla is separately selected from the group consisting of -C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-, Ci_6alkylOC(=0)Ci_6alkyl,
arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)n- (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
Rlaa is selected from the group consisting of -C(R2a)2NR3aR3b, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7Cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-,
heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl,
(ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3-7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab.
each R10 is RcRdN-;
each R11 is separately selected from the group consisting of H (hydrogen), alkoxyalkyl, Ci_6alkylOC(=0)Ci_6alkyl, Ci_6alkylC(=0)Ci_6alkyl, aryl(CH2)„- arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN(CH2)n-, (RcRdN)alkyl, and Ci_6alkyl optionally substituted with up to 9 halo;
each RcRdN is separately selected, wherein Rc and Rd are each separately selected from hydrogen, alkoxyC(=0)-, Ci_6alkyl, Ci_6alkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-, wherein the alkyl part of arylalkyl, arylalkylC(=0)-, heterocyclylalkyl, and heterocyclylalkylC(=0)- are each optionally substituted with one ReRfN- group; and wherein the aryl part of arylalkyl, arylalkylC(=0)-, arylC(=0)-, and arylsulfonyl, and the heterocyclyl part of heterocyclylalkyl, heterocyclylalkylC(=0)-, and heterocyclylC(=0)- are each optionally substituted with up to three substituents each independently selected from the group consisting of cyano, halo, nitro, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each ReRfN is separately selected, wherein Re and Rf are each separately selected from hydrogen, Ci_6alkyl, aryl, arylalkyl, cycloalkyl, (cyclolalkyl)alkyl, heterocyclyl, heterocyclylalkyl, (RxRyN)alkyl, and (RxRyN)C(=0)-;
each RxRyN is separately selected, wherein Rx and Ry are each separately selected from hydrogen, Ci_6alkylOC(=0)-, Ci_6alkyl, Ci_6alkylC(=0)-, aryl, arylalkyl, cycloalkyl, and heterocyclyl;
each C(R2a)2 is separately selected, wherein each R2a is separately selected from the group consisting of hydrogen, Ci_6alkyl optionally substituted with up to 9 halo, aryl(CH2)n-, and heteroaryl(CH2)n-, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo and Ci_6alkyl optionally substituted with up to 9
each R3a is separately selected from the group consisting of hydrogen, and optionally substituted Ci_6alkyl;
each R3b is separately selected from the group consisting of optionally substituted Ci_6alkyl, heteroaryl, -(CH2)nC(=0)NR4aR4b, -(CH2)nC(=0)OR5a, and -(CH2)nC(=0)R6a said heteroaryl optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R4aR4bN is separately selected, wherein R4a and R4b are each separately selected from the group consisting of hydrogen, optionally substituted Ci_6alkyl, and aryl(CH2)n-;
each R5a is separately selected from the group consisting of optionally substituted Ci_6alkyl, and aryl(CH2)„-;
each R6a is separately selected from the group consisting of optionally substituted Ci_6alkyl, and aryl(CH2)n-;
each A1 is separately selected from the group consisting of C2_6alkenyl, Ci_6alkyl, and -(CH2)n-0-(CH2)m-, each optionally substituted with one or more
R2;
each R2 is separately selected, wherein R2 is selected from the group consisting of protium, halo, hydroxy, Ci_6alkoxy, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, alkyoxyalkyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, heterocyclylalkyl, (ReRfN)alkyl, RaRbN-, said Ci_6alkyl optionally substituted with one or more halo, -OR2b, -C(=0)OR2b, -
C(=0)NHR2b, -NHC (=NH)NHR2b , -NHR2b, SR2b, imidazolyl, indolyl, -SCH3, phenyl, and 4-hydroxyphenyl, said Ci_6alkoxy, alkoxyalkyl, aryl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, arylalkyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (ReRfN)alkyl each optionally substituted with one or more R4, or optionally two vicinal R2 and the carbons to which they are attached are together a fused three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups;
R2b is selected from the group consisting of hydrogen, Ci_6alkyl, C2_6alkenyl, C2-6alkynyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl;
each R2c is selected from the group consisting of hydrogen, Ci_6alkyl, C2_6alkenyl, C2-6alkynyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl, said alkyl optionally substituted with ReRfN-, alkoxy, or Ci_6alkylS-;
each RaRbN is separately selected, wherein Ra and Rb are each separately selected from the group consisting of hydrogen, C2_6alkenyl, and Ci_6alkyl;
L 4 is selected from the group consisting of -J 2 -(L 5 )S-(J 2 )S-(L 5 )S-J 2 - -J 2 -
, -C(=0)-, O (oxygen), - o
OC(R2)2-, ¾ H s -C(CF3)2NR2c-, NH, and -(CH=CH)-;
each J2 is phenyl optionally substituted with one or more R15;
each R14 is separately selected from the group consisting of hydroxy, Ci_6alkoxy optionally substituted with up to 9 fluoro, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R15 is separately selected from the group consisting of halo, hydroxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, R9, RxRyN, RxRyNC(=0), RxRyNCi_6alkyl, heteroaryl, aryl, Ci_6alkyl optionally substituted with up to 9 halo, Ci_6alkyl substituted with up to 5 hydroxy, Ci_6alkoxy optionally substituted with up to 9 halo, Ci_6haloalkyl, RaRbN-, (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl substituted with up to 5 hydroxy, said substituent aryl and heteroaryl are each optionally substituted with one or more R14, or optionally
two vicinal R15 and the carbons to which they are attached are together a fused three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups, or optionally two geminal R15 and the carbon to which they are attached are together a three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups, or optionally two geminal R15 are together oxo; each L5 is separately selected from the group consisting of
X -C(CF3)2NR2c- -C(R2)2- -C(=0)-, O
(oxygen), NH, and -(CH=CH)-;
each A is separately selected from the group consisting of CR3 and N (nitrogen);
L6 is selected from the group consisting of
L7 is selected from the group consisting of
C(R2)20-, -C(=0)-, O (oxygen), NH, and -(CH=CH)-;
each X4 is separately selected from the group consisting of CR4 and N
(nitrogen);
each Y4 is separately selected from the group consisting of C(R4)2, NR4, O (oxygen), and S (sulfur);
each X9 is separately selected from the group consisting of CH and N (nitrogen);
each Y10 is separately selected from the group consisting of -CH2- and -
NH-;
each m separately is 1 or 2;
each n separately is 0, 1 or 2;
each p separately is 1, 2, 3 or 4;
each q separately is 1, 2, 3, 4 or 5;
each r separately is 0, 1, 2, 3, or 4;
each s separately is 0 or 1 ;
each R3 is separately selected from the group consisting of hydrogen, Ci_6alkoxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, hydroxy, RaRbN- (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy;
each R4 is separately selected from the group consisting of H (hydrogen), Ci_6alkoxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, Ci_6haloalkyl, hydroxy, RaRbN-, (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy, or optionally two geminal R4 are together oxo;
R6 is Ci_6alkyl optionally substituted with up to 9 halo;
R9 is selected from the group consisting of hydrogen and -C(=0)R9a;
R9a is selected from the group consisting of -NR9bR9c, -OR9d, Ci_ Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
R9b is selected from the group consisting of hydrogen, Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
R9c is selected from the group consisting of Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl; and
R9d is selected from the group consisting of Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl.
[0023] In some embodiments, the compound of Formula XVI has the structure:
or a pharmaceutically acceptable salt thereof.
[0024] Some embodiments provide a compound having the structure of Formula XVIII:
Q 6
I
|_16 |_14
L17_L1£
Q 7
XVIII
or a pharmaceutically acceptable salt thereof,
wherein:
each Y2 is selected from O (oxygen), S (sulfur), S(O), S02, NR2, and C(R2)2 with the proviso that when X2 is null Y2 is C(R2)2;
each X10 is (C(R2)2)q;
each Y11 is separately selected from the group consisting of -0(C(R2)2)n-, -S(C(R2)2)„-, -S(0)(C(R2)2)n-, -S02(C(R2)2)n-, -NR2(C(R2)2)„-, and (C(R2)2)q;
12 13 12 13
each R R TSi is separately selected, wherein R and R are each separately selected from hydrogen, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3-7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab. or R12R13N is a heterocyclyl linked through a ring nitrogen atom optionally substituted with one or more of oxo, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-
Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab.
each Rlab is separately selected from the group consisting of -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80,
arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)n-, (RcRdN)(CH=CH)m- (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R80 is separately selected from the group consisting of hydrogen, alkoxyalkyl, Ci_6alkyl, C3-7cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and (ReRfN)alkyl, said alkoxyalkyl, Ci_6alkyl, C3_7cycloalkyl, aryl, arylalkyl,
heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (ReRfN)alkyl are each optionally substituted with one or more Rlac;
each Rlac is separately selected from the group consisting of -C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-,
Ci_6alkylC(=0)Ci_6alkyl, aryl, aryl(CH2)„-, aryl(CH2)nO-, aryl(CH=CH)m-, arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)„-, (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each Y14 is separately selected from the group consisting of -C(=0)-, - S(=0)-, -C(=S)-, -S(=0)2-, -C(=0)0-, -C(=0)NR2\ -S(=0)2NR2\ - C(=0)NR2cC(=0)-, and -C(CF3)2NR2c-,
each R1 is separately selected from the group consisting of Rlaa, RlaC(=0)- and RlaC(=S)-;
each Rla is separately selected from the group consisting of -C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-,
Ci_6alkylC(=0)Ci_6alkyl, aryl, aryl(CH2)„-, aryl(CH2)nO-, aryl(CH=CH)m-, arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)„-, (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each Rlaa is separately selected from the group consisting of -C(R2a)2NR3aR3b, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl,
C2_6alkenyl, C2_6alkynyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3-7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab.
each R10 is RcRdN-;
each R11 is separately selected from the group consisting of H (hydrogen), alkoxyalkyl, Ci_6alkylOC(=0)Ci_6alkyl, Ci_6alkylC(=0)Ci_6alkyl, aryl(CH2)„- arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN(CH2)n-, (RcRdN)alkyl, and Ci_6alkyl optionally substituted with up to 9 halo;
each RcRdN is separately selected, wherein Rc and Rd are each separately selected from hydrogen, alkoxyC(=0)-, Ci_6alkyl, Ci_6alkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-, wherein the alkyl part of arylalkyl, arylalkylC(=0)-, heterocyclylalkyl, and heterocyclylalkylC(=0)- are each optionally substituted with one ReRfN- group; and wherein the aryl part of arylalkyl, arylalkylC(=0)-, arylC(=0)-, and arylsulfonyl, and the heterocyclyl part of heterocyclylalkyl, heterocyclylalkylC(=0)-, and heterocyclylC(=0)- are each optionally substituted with up to three substituents each independently selected from the group consisting of cyano, halo, nitro, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each ReRfN is separately selected, wherein Re and Rf are each separately selected from hydrogen, Ci_6alkyl, aryl, arylalkyl, cycloalkyl, (cyclolalkyl)alkyl, heterocyclyl, heterocyclylalkyl, (RxRyN)alkyl, and (RxRyN)C(=0)-;
each RxRyN is separately selected, wherein Rx and Ry are each separately selected from hydrogen, Ci_6alkylOC(=0)-, Ci_6alkyl, Ci_6alkylC(=0)-, aryl, arylalkyl, cycloalkyl, and heterocyclyl;
each C(R2a)2 is separately selected, wherein each R2a is separately selected from the group consisting of hydrogen, Ci_6alkyl optionally substituted with up to 9 halo, aryl(CH2)n-, and heteroaryl(CH2)n-, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo and Ci_6alkyl optionally substituted with up to 9
each R3a is separately selected from the group consisting of hydrogen, and optionally substituted Ci_6alkyl;
each R3b is separately selected from the group consisting of optionally substituted Ci_6alkyl, heteroaryl, -(CH2)nC(=0)NR4aR4b, -(CH2)nC(=0)OR5a, and -(CH2)nC(=0)R6a said heteroaryl optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R4aR4bN is separately selected, wherein R4a and R4b are each separately selected from the group consisting of hydrogen, optionally substituted Ci_6alkyl, and aryl(CH2)n-;
each R5a is separately selected from the group consisting of optionally substituted Ci_6alkyl, and aryl(CH2)„-;
each R6a is separately selected from the group consisting of optionally substituted Ci_6alkyl, and aryl(CH2)n-;
each A1 is separately selected from the group consisting of C2_6alkenyl, Ci_6alkyl, and -(CH2)n-0-(CH2)m-, each optionally substituted with one or more
R2;
each R2 is separately selected, wherein R2 is selected from the group consisting of hydrogen, halo, hydroxy, Ci_6alkoxy, Ci_6alkyl, C2_6alkenyl,
C2-6alkynyl, alkyoxyalkyl, C3_7Cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, heterocyclylalkyl, (ReRfN)alkyl, RaRbN-, said Ci_6alkyl optionally substituted with one or more halo, -OR2b, -C(=0)OR2b, - C(=0)NHR2b, -NHC (=NH)NHR2b , -NHR2b, SR2b, imidazolyl, indolyl, -SCH3, phenyl, and 4-hydroxyphenyl, said Ci_6alkoxy, alkoxyalkyl, aryl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, arylalkyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (ReRfN)alkyl each optionally substituted with one or more R4, or optionally two vicinal R2 and the carbons to which they are attached are together a fused three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups;
R2b is selected from the group consisting of hydrogen, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl;
each R2c is selected from the group consisting of hydrogen, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl, said alkyl optionally substituted with ReRfN-, alkoxy, or Ci_6alkylS-;
each RaRbN is separately selected, wherein Ra and Rb are each separately selected from the group consisting of hydrogen, C2_6alkenyl, and Ci_6alkyl;
L14 is -Jn-J2-J12-;
J2 is aryl, heteroaryl, heterocyclyl, cycloalkyl, cycloalkenyl, or polycyclic hydrocarbon, each optionally substituted with one or more R15;
J11 is C(R4)2, NR4, O (oxygen), or S (sulfur);
J12 is -C(=0)-, S(O), S02, or -C(=NR4)-;
each R14 is separately selected from the group consisting of hydroxy, Ci_6alkoxy optionally substituted with up to 9 fluoro, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R15 is separately selected from the group consisting of halo, hydroxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, R9, RxRyN, RxRyNC(=0), RxRyNCi_6alkyl, heteroaryl, aryl, Ci_6alkyl optionally substituted with up to 9 halo, Ci_6alkyl substituted with up to 5 hydroxy, Ci_6alkoxy optionally substituted with up to 9 halo, Ci_6haloalkyl, RaRbN-, (RaRbN)alkyl,
(RaRbN)C(=0)-, Ci_6alkyl substituted with up to 5 hydroxy, said substituent aryl and heteroaryl are each optionally substituted with one or more R14, or optionally two vicinal R15 and the carbons to which they are attached are together a fused three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups, or optionally two geminal R15 and the carbon to which they are attached are together a three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups, or optionally two geminal R15 are together oxo;
16 is selected from the group consisting of
C(R2)2, -C(R2)20-, -C(=0)-, O (oxygen), NH, and -(CH=CH)-;
each A is separately selected from the group consisting of CR3 and N (nitrogen);
each R3 is separately selected from the group consisting of hydrogen, Ci_6alkoxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, hydroxy, RaRbN- (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy;
L17 is selected from the group consisting of aryl, heteroaryl, heterocyclyl, cycloalkyl, cycloalkenyl, or polycyclic hydrocarbon, each optionally substituted with one or more R 15.
each X4 is separately selected from the group consisting of CR4 and N
(nitrogen);
each Y4 is separately selected from the group consisting of C(R4)2, NR4, O (oxygen), and S (sulfur);
each X9 is separately selected from the group consisting of CH and N (nitrogen);
each Y10 is separately selected from the group consisting of -CH2- and -
NH-;
each m separately is 1 or 2;
each n separately is 0, 1 or 2;
each p separately is 1, 2, 3 or 4;
each q separately is 1, 2, 3, 4 or 5;
each r separately is 0, 1, 2, 3, or 4;
each s separately is 0 or 1 ;
each R4 is separately selected from the group consisting of H (hydrogen), Ci_6alkoxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, Ci_6haloalkyl, hydroxy, RaRbN-, (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy, or optionally two geminal R4 are together oxo;
R9 is selected from the group consisting of hydrogen and -C(=0)R9a;
R9a is selected from the group consisting of -NR9bR9c, -OR9d, Ci_ Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
R9b is selected from the group consisting of hydrogen, Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
R9c is selected from the group consisting of Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl; and
R9d is selected from the group consisting of Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl.
[0025] In some embodiments, the compound of Formula XVIII has the structure of Formula XVIIIa:
XVIIIa
or a pharmaceutically acceptable salt thereof,
B can be aryl, heteroaryl, or cycloalkenyl, each optionally substituted with one or more R15;
B11 can be a heterocyclyl, cycloalkyl, or polycyclic hydrocarbon, each optionally substituted with one or more R15; and
each R16 can separately be hydrogen or R15.
[0026] In some embodiments of Formula XVIIIa,
B can be aryl, or heteroaryl;
J11 can be NR4; and
J12 can be -C(=0)-.
[0027] In some embodiments of Formula XVIIIa, B can be phenyl.
each X14 can separately be selected from the group consisting of CR17 and N (nitrogen);
Y14 can be selected from the group consisting of NR17, O (oxygen), and S (sulfur);
each R17 can be separately hydrogen or R15.
χ9_γ10
L18 can be ¾ X9 * ; and
R4 can be hydrogen.
[0030] som embodiments of Formula XVIIIa,
[0031] In some embodiments, the compound of Formula XVIII has the structure:
or a pharmaceutically acceptable salt thereof.
[0033] Some embodiments provide a pharmaceutical composition comprising a pharmaceutically acceptable excipient and a compound of Formulas XV, XVI, XVII or XVIII.
[0034] Some embodiments provide a method of treating HCV infection in an individual, the method comprising administering to the individual an effective amount of a compound of Formulas XV, XVI, XVII or XVIII or a pharmaceutical composition comprising a pharmaceutically acceptable excipient and a compound of Formulas XV, XVI, XVII or XVIII.
[0035] Some embodiments provide a method of treating HCV infection in an individual, the method comprising administering to the individual an effective amount of a compound of Formulas XV, XVI, XVII or XVIII or a pharmaceutical composition comprising a pharmaceutically acceptable excipient and a compound of Formulas XV, XVI, XVII or XVIII In some embodiments, the method further comprises identifying a subject suffering from a hepatitis C infection.
[0036] Some embodiments provide a method of treating liver fibrosis in an individual, the method comprising administering to the individual an effective amount of a compound of Formulas XV, XVI, XVII or XVIII or a pharmaceutical composition comprising a pharmaceutically acceptable excipient and a compound of Formulas XV, XVI, XVII or XVIII. In some embodiments, the method further comprises identifying a subject suffering from a hepatitis C infection.
[0037] Some embodiments provide a method of increasing liver function in an individual having a hepatitis C virus infection, the method comprising administering to the individual an effective amount of a compound of Formulas XV, XVI, XVII or XVIII or a pharmaceutical composition comprising a pharmaceutically acceptable excipient and a compound of Formulas XV, XVI, XVII or XVIII. In some embodiments, the method further comprises identifying a subject suffering from a hepatitis C infection.
DETAILED DESCRIPTION
Definitions
[0038] As used herein, common organic abbreviations are defined ί
Ac Acetyl
Ac20 Acetic anhydride
aq. Aqueous
Bn Benzyl
Bz Benzoyl
BOC or Boc tert-Butoxycarbonyl
Bu n-Butyl
cat. Catalytic
Cbz Carbobenzyloxy
CDI 1 , 1 ' -carbonyldiimidazole
Cy (c-C6Hii) Cyclohexyl
°C Temperature in degrees Centigrade
DBU l ,8-Diazabicyclo[5.4.0]undec-7-ene
DCE 1 ,2-Dichloroethane
DCM methylene chloride
DIEA Diisopropylethylamine
DMA Dimethylacetamide
DME Dimethoxyethane
DMF N^-Dimethylformamide
DMSO Dimethylsulfoxide
Et Ethyl
EtOAc Ethyl acetate
g Gram(s)
h Hour (hours)
HATU 2-(lH-7-azabenzotriazol-l-yl)-l , l ,3,3-tetramethyl uranium hexafluorophosphate
ΗΟΒΤ N-Hydroxybenzotriazole
iPr Isopropyl
LCMS Liquid chromatography-mass spectrometry
LDA Lithium diisopropylamide
mCPBA meta-Chloroperoxybenzoic Acid
MeOH Methanol
MeCN Acetonitrile
mL Milliliters)
MTBE Methyl tertiary-butyl ether
NH4OAc Ammonium acetate
PG Protecting group
Pd/C Palladium on activated carbon
Ph Phenyl
ppt Precipitate
RCM Ring closing metathesis
rt Room temperature
sBuLi sec-Butylithium
TEA Triethylamine
TCDI 1 , Γ-Thiocarbonyl diimidazole
Tert, t tertiary
TFA Trifluoracetic acid
THF Tetrahydrofuran
TLC Thin-layer chromatography
TMEDA Tetramethylethylenediamine
Microliter(s)
[0039] The terms "individual," "host," "subject," and "patient" are used interchangeably herein, and refer to a mammal, including, but not limited to, primates, including simians and humans.
[0040] As used herein, the term "liver function" refers to a normal function of the liver, including, but not limited to, a synthetic function, including, but not limited to, synthesis of proteins such as serum proteins (e.g., albumin, clotting factors, alkaline phosphatase, aminotransferases (e.g., alanine transaminase, aspartate transaminase), 5'- nucleosidase, γ-glutaminyltranspeptidase, etc.), synthesis of bilirubin, synthesis of cholesterol, and synthesis of bile acids; a liver metabolic function, including, but not limited to, carbohydrate metabolism, amino acid and ammonia metabolism, hormone metabolism, and lipid metabolism; detoxification of exogenous drugs; a hemodynamic function, including splanchnic and portal hemodynamics; and the like.
[0041] The term "sustained viral response" (SVR; also referred to as a "sustained response" or a "durable response"), as used herein, refers to the response of an individual to a treatment regimen for HCV infection, in terms of serum HCV titer. Generally, a "sustained viral response" refers to no detectable HCV R A (e.g., less than about 500, less than about 200, or less than about 100 genome copies per milliliter serum) found in the patient's serum for a period of at least about one month, at least about two months, at least about three months, at least about four months, at least about five months, or at least about six months following cessation of treatment.
[0042] "Treatment failure patients" as used herein generally refers to HCV- infected patients who failed to respond to previous therapy for HCV (referred to as "non- responders") or who initially responded to previous therapy, but in whom the therapeutic response was not maintained (referred to as "relapsers"). The previous therapy generally can include treatment with IFN-a monotherapy or IFN-a combination therapy, where the combination therapy may include administration of IFN-a and an antiviral agent such as ribavirin.
[0043] As used herein, the terms "treatment," "treating," and the like, refer to obtaining a desired pharmacologic and/or physiologic effect. The effect may be prophylactic in terms of completely or partially preventing a disease or symptom thereof and/or may be therapeutic in terms of a partial or complete cure for a disease and/or adverse affect attributable to the disease. "Treatment," as used herein, covers any treatment of a disease in a mammal, particularly in a human, and includes: (a) preventing the disease from occurring in a subject which may be predisposed to the disease but has not yet been diagnosed as having it; (b) inhibiting the disease, i.e., arresting its development; and (c) relieving the disease, i.e., causing regression of the disease.
[0044] As used herein, the term "alkyl" refers to a branched or unbranched fully saturated acyclic aliphatic hydrocarbon group (i.e. composed of carbon and hydrogen containing no double or triple bonds). In some embodiments, alkyls may be substituted or unsubstituted. Alkyls include, but are not limited to, methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tertiary butyl, pentyl, hexyl, and the like, each of which may be optionally substituted in some embodiments.
[0045] As used herein, the term "heteroalkyl" refers to a branched or unbrached fully saturated acyclic aliphatic hydrocarbon group containing one or more heteroatoms in the carbon back bone (i.e., an alkyl group in which one or more carbon
atoms is replaced with a heteroatom). In some embodiments, heteroalkyls may be substituted or unsubstituted. Heteroalkyls include, but are not limited to, ethers, thioethers, and alkyl-amino-alkyls.
[0046] The term "halo" used herein refers to fluoro, chloro, bromo, or iodo.
[0047] The term "alkoxy" used herein refers to straight or branched chain alkyl radical covalently bonded to the parent molecule through an— O— linkage. In some embodiments, alkoxys may be substituted or unsubstituted. Examples of alkoxy groups include, but are not limited to, methoxy, ethoxy, propoxy, isopropoxy, butoxy, n-butoxy, sec-butoxy, t-butoxy and the like.
[0048] The term "alkenyl" used herein refers to a monovalent straight or branched chain radical of from two to twenty carbon atoms containing at least one carbon-carbon double bond including, but not limited to, 1-propenyl, 2-propenyl, 2- methyl-l-propenyl, 1-butenyl, 2-butenyl, and the like. In some embodiments, alkenyls may be substituted or unsubstituted.
[0049] The term "alkynyl" used herein refers to a monovalent straight or branched chain radical of from two to twenty carbon atoms containing at least one carbon-carbon triple bond including, but not limited to, 1-propynyl, 1-butynyl, 2-butynyl, and the like. In some embodiments, alkynyls may be substituted or unsubstituted
[0050] The term "aryl" used herein refers to a homocyclic aromatic radical having one ring, two appended rings, or multiple fused rings. Examples of aryl groups include, but are not limited to, phenyl, naphthyl, biphenyl, phenanthrenyl, naphthacenyl, and the like. In some embodiments, aryls may be substituted or unsubstituted.
[0051] The term "cycloalkyl" used herein refers to a saturated aliphatic ring system radical having three to twenty carbon atoms including, but not limited to, cyclopropyl, cyclopentyl, cyclohexyl, cycloheptyl, and the like. In some embodiments, cycloalkyls may be substituted or unsubstituted.
[0052] The term "cycloalkenyl" used herein refers to an aliphatic ring system radical having three to twenty carbon atoms having at least one carbon-carbon double bond in the ring. Examples of cycloalkenyl groups include, but are not limited to, cyclopropenyl, cyclopentenyl, cyclohexenyl, cycloheptenyl, and the like. In some embodiments, cycloalkenyls may be substituted or unsubstituted.
[0053] The term "polycycloalkyl" used herein refers to saturated aliphatic ring system radical having at least two rings that are fused with or without bridgehead
carbons. Examples of polycycloalkyl groups include, but are not limited to, bicyclo[4.4.0]decanyl, bicyclo[2.2.1]heptanyl, adamantyl, norbornyl, and the like.
[0054] The term "polycycloalkenyl" used herein refers to aliphatic ring system radical having at least two rings that are fused with or without bridgehead carbons in which at least one of the rings has a carbon-carbon double bond. Examples of polycycloalkenyl groups include, but are not limited to, norbornylenyl, 1,1 '- bicyclopentenyl, and the like.
[0055] The term "polycyclic hydrocarbon" used herein refers to a ring system radical in which all of the ring members are carbon atoms. Polycyclic hydrocarbons can be aromatic or can contain less than the maximum number of non-cumulative double bonds. Examples of polycyclic hydrocarbon include, but are not limited to, naphthyl, dihydronaphthyl, indenyl, fluorenyl, and the like.
[0056] The term "heterocyclic" or "heterocyclyl" or "heterocycloalkyl" used herein refers to a cyclic ring system radical having at least one non-aromatic ring in which one or more ring atoms are not carbon, namely heteroatom. Monocyclic "heterocyclic" or "heterocyclyl" moieties are non-aromatic. Bicyclic "heterocyclic" or "heterocyclyl" moieties include one non-aromatic ring wherein at least one heteroatom is present in a ring. Tricyclic "heterocyclic" or "heterocyclyl" moieties include at least one non-aromatic ring wherein at least one heteroatom is present in a ring. Examples of heterocyclic groups include, but are not limited to, morpholinyl, tetrahydrofuranyl, dioxolanyl, pyrolidinyl, oxazolyl, pyranyl, pyrrolyl, isoindoline and the like.
[0057] The term "heteroaryl" used herein refers to an aromatic ring system radical in which one or more ring atoms are not carbon, namely heteroatom, having one ring or multiple fused rings. In fused ring systems, the one or more heteroatoms may be present in only one of the rings. Examples of heteroaryl groups include, but are not limited to, benzothiazyl, benzoxazyl, quinazolinyl, quinolinyl, isoquinolinyl, quinoxalinyl, pyridinyl, pyrrolyl, oxazolyl, indolyl, and the like. In some embodiments, heteroaryls may be substituted or unsubstituted.
[0058] The term "heteroatom" used herein refers to, for example, oxygen, sulfur and nitrogen.
[0059] The term "arylalkyl" used herein refers to one or more aryl groups appended to an alkyl radical. Examples of arylalkyl groups include, but are not limited to, benzyl, phenethyl, phenpropyl, phenbutyl, and the like. In some embodiments,
arylalkyls may be substituted or unsubstituted, and can be substituted on either the aryl or alkyl portion or on both.
[0060] The term "cycloalkylalkyl" used herein refers to one or more cycloalkyl groups appended to an alkyl radical. Examples of cycloalkylalkyl include, but are not limited to, cyclohexylmethyl, cyclohexylethyl, cyclopentylmethyl, cyclopentylethyl, and the like. In some embodiments, cycloalkylalkyls may be substituted or unsubstituted.
[0061] The term "heteroarylalkyl" used herein refers to one or more heteroaryl groups appended to an alkyl radical. Examples of heteroarylalkyl include, but are not limited to, pyridylmethyl, furanylmethyl, thiopheneylethyl, and the like. In some embodiments, heteroarylalkyls may be substituted or unsubstituted, and can be substituted on either the heteroaryl or alkyl portion or on both.
[0062] The term "heterocyclylalkyl" used herein refers to one or more heterocyclyl groups appended to an alkyl radical. Examples of heterocyclylalkyl include, but are not limited to, morpholinylmethyl, morpholinylethyl, morpholinylpropyl, tetrahydrofuranylmethyl, pyrrolidinylpropyl, and the like.
[0063] he term "aryloxy" used herein refers to an aryl radical covalently bonded to the parent molecule through an— O— linkage.
[0064] The term "alkylthio" used herein refers to straight or branched chain alkyl radical covalently bonded to the parent molecule through an — S— linkage. Examples of alkylthio groups include, but are not limited to, methanesulfide, ethanesulfide, propanesulfide, isopropanesulfide, butanesulfide, n-butanesulfide, sec- butanesulfide, tert-butanesulfide and the like.
[0065] The term "arylthio" used herein refers to an aryl radical covalently bonded to the parent molecule through an— S— linkage.
[0066] The term "alkylamino" used herein refers to nitrogen radical with one or more alkyl groups attached thereto. Thus, monoalkylamino refers to nitrogen radical with one alkyl group attached thereto and dialkylamino refers to nitrogen radical with two alkyl groups attached thereto.
[0067] The term "cyanoamino" used herein refers to nitrogen radical with nitrile group attached thereto.
[0068] The term "carbamyl" used herein refers to RNHCOO-.
[0069] The term "keto" and "carbonyl" used herein refers to C=0.
[0070] The term "carboxy" used herein refers to -COOH.
[0071] The term "sulfamyl" used herein refers to -S02NH2.
[0072] The term "sulfonyl" used herein refers to -S02-.
[0073] The term "sulfmyl" used herein refers to -SO-.
[0074] The term "thiocarbonyl" used herein refers to C=S.
[0075] The term "thiocarboxy" used herein refers to CSOH.
[0076] The term "sulfonamide" used herein refers to -S02NR'2 where each R' is individually selected from H (hydrogen), Ci-C6 alkyl, C3-C7 cycloalkyl, arylalkyl and aryl optionally substituted with Ci-C6 alkyl.
[0077] The term "ester" used herein refers to -COOR' where R' is selected from Ci-C6 alkyl, C3-C7 cycloalkyl, arylalkyl and aryl optionally substituted with Ci-C6 alkyl.
[0078] The term "C-amide" used herein refers to -C(=0)NR'2 where each R' is individually selected from H (hydrogen), Ci-C6 alkyl, C3-C7 cycloalkyl, arylalkyl and aryl optionally substituted with Ci-C6 alkyl.
[0079] The term "N-amide" used herein refers to -NR'C(=0)R' where each R' is individually selected from H (hydrogen), Ci-C6 alkyl, C3-C7 cycloalkyl, arylalkyl and aryl optionally substituted with Ci-C6 alkyl.
[0080] The term "N-carbamate" used herein refers to -NR'C(=0)OR' where each R' is individually selected from H (hydrogen), Ci-C6 alkyl, C3-C7 cycloalkyl, arylalkyl and aryl optionally substituted with Ci-C6 alkyl.
[0081] The term "O-carbamate" used herein refers to -OC(=0)NR'2 where each R' is individually selected from H (hydrogen), Ci-C6 alkyl, C3-C7 cycloalkyl, arylalkyl and aryl optionally substituted with Ci-C6 alkyl.
[0082] The term "urea" used herein refers to -NR'C(=0)NR'2 where each R' is individually selected from H (hydrogen), Ci-C6 alkyl, C3-C7 cycloalkyl, arylalkyl and aryl optionally substituted with Ci-C6 alkyl.
[0083] As used herein, a radical indicates a species with one or more, unpaired electron such that the species containing the radical can be covalently bonded to one or more other species. Hence, in this context, a radical is not necessarily a free radical. Rather, a radical indicates a specific portion of a larger molecule. The term "radical" can be used interchangeably with the term "moiety" or "group."
[0084] As used herein, a substituted group is derived from the unsubstituted parent structure in which there has been an exchange of one or more hydrogen atoms for another atom or group. When substituted, the substituent group(s) is (are) one or more group(s) individually and independently selected from Ci-C6 alkyl, Ci-C6 alkenyl, Ci-C6 alkynyl, C3-C7 cycloalkyl (optionally substituted with halo, alkyl, alkoxy, carboxyl, haloalkyl, CN, -S02-alkyl, -CF3, and -OCF3), cycloalkyl geminally attached, Ci-C6 heteroalkyl, C3-Ci0 heterocycloalkyl (e.g., tetrahydrofuryl) (optionally substituted with halo, alkyl, alkoxy, carboxyl, CN, -S02-alkyl, -CF3, and -OCF3), aryl (optionally substituted with halo, alkyl, aryl optionally substituted with Ci-C6 alkyl, arylalkyl, alkoxy, carboxyl, CN, -S02-alkyl, -CF3, and -OCF3), arylalkyl (optionally substituted with halo, alkyl, alkoxy, aryl, carboxyl, CN, -S02-alkyl, -CF3, and -OCF3), heteroaryl (optionally substituted with halo, alkyl, alkoxy, aryl, aralkyl, carboxyl, CN, -S02-alkyl, - CF3, and -OCF3), halo (e.g., chloro, bromo, iodo and fluoro), cyano, hydroxy, -CF3, Ci- C6 alkoxy, aryloxy, sulfhydryl (mercapto), halo(Ci-Ce)alkyl, Ci-C6 alkylthio, arylthio, mono- and di-(Ci-C6)alkyl amino, quaternary ammonium salts, amino(Ci-C6)alkoxy, hydroxy(Ci-C6)alkylamino, amino(Ci-C6)alkylthio, cyanoamino, nitro, carbamyl, keto (oxy), carbonyl, carboxy, glycolyl, glycyl, hydrazino, guanyl, sulfamyl, sulfonyl, sulfinyl, thiocarbonyl, thiocarboxy, sulfonamide, ester, C-amide, N-amide, N-carbamate, O- carbamate, and urea. The protecting groups that can form the protective derivatives of the above substituents are known to those of skill in the art and can be found in references such as Greene and Wuts Protective Groups in Organic Synthesis; John Wiley and Sons: New York, 1999. Wherever a substituent is described as "optionally substituted" that substituent can be substituted with the above substituents.
[0085] Asymmetric carbon atoms may be present in the compounds described. All such isomers, including diastereomers and enantiomers, as well as the mixtures thereof are intended to be included in the scope of the recited compound. In certain cases, compounds can exist in tautomeric forms. All tautomeric forms are intended to be included in the scope. Likewise, when compounds contain an alkenyl or alkenylene group, there exists the possibility of cis- and trans- isomeric forms of the compounds. Both cis- and trans- isomers, as well as the mixtures of cis- and trans- isomers, are contemplated. Thus, reference herein to a compound includes all of the aforementioned isomeric forms unless the context clearly dictates otherwise.
[0086] Various forms are included in the embodiments, including polymorphs, solvates, hydrates, conformers, salts, and prodrug derivatives. A polymorph is a composition having the same chemical formula, but a different structure. A solvate is a composition formed by solvation (the combination of solvent molecules with molecules or ions of the solute). A hydrate is a compound formed by an incorporation of water. A conformer is a structure that is a conformational isomer. Conformational isomerism is the phenomenon of molecules with the same structural formula but different conformations (conformers) of atoms about a rotating bond. Salts of compounds can be prepared by methods known to those skilled in the art. For example, salts of compounds can be prepared by reacting the appropriate base or acid with a stoichiometric equivalent of the compound. A prodrug is a compound that undergoes biotransformation (chemical conversion) before exhibiting its pharmacological effects. For example, a prodrug can thus be viewed as a drug containing specialized protective groups used in a transient manner to alter or to eliminate undesirable properties in the parent molecule. Thus, reference herein to a compound includes all of the aforementioned forms unless the context clearly dictates otherwise.
[0087] The term "pharmaceutically acceptable salt," as used herein, and particularly when referring to a pharmaceutically acceptable salt of a compound, including a compound of Formulas XV, XVI, XVII or XVIII, as produced and synthesized by the methods disclosed herein, refers to any pharmaceutically acceptable salts of a compound, and preferably refers to an acid addition salt of a compound. With respect to compounds synthesized by the method of this embodiment that contain a basic nitrogen, the preferred examples of pharmaceutically acceptable salts are acid addition salts of pharmaceutically acceptable inorganic or organic acids, including, but not limited to, hydrohalic, sulfuric, phosphoric, aliphatic or aromatic carboxylic, or sulfonic acid. Examples of pharmaceutically acceptable inorganic or organic acids as a component of an addition salt, include but are not limited to, hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid acetic acid, succinic acid, lactic acid, malic acid, tartaric acid, citric acid, ascorbi acid c, nicotinic acid, methanesulfonic acid, p-toluensulfonic acid or naphthalenesulfonic acid acid. With respect to compounds synthesized by the methods of this embodiment that contain an acidic functional group, the preferred examples of pharmaceutically acceptable salts include, but are not limited to, alkali metal salts (sodium or potassium), alkaline earth metal salts (calcium or magnesium), or ammonium
salts derived from ammonia or from pharmaceutically acceptable organic amines, for example C1-C7 alkylamine, cyclohexylamine, triethanolamine, ethylenediamine or tris- (hydroxymethyl)-aminomethane .
[0088] Isotopes may be present in the compounds described. Each chemical element as represented in a compound structure may include any isotope of said element. For example, in a compound structure a hydrogen atom may be explicitely disclosed or understood to be present in the compound. At any position of the compound that a hydrogen atom may be present, the hydrogen atom can be any isotope of hydrogen, including but not limited to hydrogen- 1 (protium) and hydrogen-2 (deuterium). Thus, reference herein to a compound encompasses all potential isotopic forms unless the context clearly dictates otherwise.
[0089] Whereever a substituent as depicted as a di-radical (i.e., has two points of attachment to the rest of the molecule), it is to be understood that the substituent can be attached in any directional configuration unless otherwise indicated. Thus, for example, a substituent depicted as -AE- or ¾ A\ E A includes the substituent being oriented such that the A is attached at the leftmost attachment point of the molecule as well as the case in which A is attached at the rightmost attachment point of the molecule.
[0090] It is to be understood that certain radical naming conventions can include either a mono-radical or a di-radical, depending on the context. For example, where a substituent requires two points of attachment to the rest of the molecule, it is understood that the substituent is a di-radical. A substituent identified as alkyl, that requires two points of attachment, includes di-radicals such as -CH2-, -CH2CH2-, - CH2CH(CH3)CH2-, and the like; a substituent depicted as alkoxy that requires two points of attachment, includes di-radicals such as -OCH2- -OCH2CH2- -OCH2CH(CH3)CH2- and the like: and a substituent depict as arylC(=0)- that requires two points of
and the like.
[0091] Where a range of values is provided, it is understood that the upper and lower limit, and each intervening value between the upper and lower limit of the range is encompassed within the embodiments.
[0092] Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which the embodiments belong. Although any methods and materials similar or equivalent to those described herein can also be used in the practice or testing of the embodiments, the preferred methods and materials are now described. All publications mentioned herein are incorporated herein by reference to disclose and describe the methods and/or materials in connection with which the publications are cited.
[0093] It must be noted that as used herein and in the appended claims, the singular forms "a," "and," and "the" include plural referents unless the context clearly dictates otherwise. Thus, for example, reference to "a method" includes a plurality of such methods and reference to "a dose" includes reference to one or more doses and equivalents thereof known to those skilled in the art, and so forth.
Compounds
[0094] The present embodiments provide compounds of Formulas XV, XVI, XVII or XVIII, as defined above, as well as pharmaceutical compositions and formulations comprising any compound of Formulas XV, XVI, XVII or XVIII. A subject compound is useful for treating HCV infection and other disorders, as discussed below.
[0095] In many embodiments, a subject compound inhibits HCV viral replication. For example, a subject compound inhibits HCV viral replication by at least about 10%, at least about 15%, at least about 20%>, at least about 25%, at least about 30%, at least about 40%, at least about 50%, at least about 60%, at least about 70%, at least about 80%, or at least about 90%, or more, compared to HCV viral replication in the absence of the compound. Whether a subject compound inhibits HCV viral replication can be determined using methods known in the art, including an in vitro viral replication assay.
Compositions
[0096] The present embodiments further provide compositions, including pharmaceutical compositions, comprising compounds of the general Formulas XV, XVI, XVII or XVIII.
[0097] A subject pharmaceutical composition comprises a subject compound; and a pharmaceutically acceptable excipient. A wide variety of pharmaceutically acceptable excipients is known in the art and need not be discussed in detail herein. Pharmaceutically acceptable excipients have been amply described in a variety of
publications, including, for example, A. Gennaro (2000) "Remington: The Science and Practice of Pharmacy," 20th edition, Lippincott, Williams, & Wilkins; Pharmaceutical Dosage Forms and Drug Delivery Systems (1999) H.C. Ansel et al, eds., 7th ed., Lippincott, Williams, & Wilkins; and Handbook of Pharmaceutical Excipients (2000) A.H. Kibbe et al., eds., 3rd ed. Amer. Pharmaceutical Assoc.
[0098] The pharmaceutically acceptable excipients, such as vehicles, adjuvants, carriers or diluents, are known in the art. Moreover, pharmaceutically acceptable auxiliary substances, such as pH adjusting and buffering agents, tonicity adjusting agents, stabilizers, wetting agents and the like, are known in the art.
[0099] In some embodiments, a compound as described herein is formulated in an aqueous buffer. Suitable aqueous buffers include, but are not limited to, acetate, succinate, citrate, and phosphate buffers varying in strengths from about 5mM to about lOOmM. In some embodiments, the aqueous buffer includes reagents that provide for an isotonic solution. Such reagents include, but are not limited to, sodium chloride; and sugars e.g., mannitol, dextrose, sucrose, and the like. In some embodiments, the aqueous buffer further includes a non-ionic surfactant such as polysorbate 20 or 80. Optionally the formulations may further include a preservative. Suitable preservatives include, but are not limited to, a benzyl alcohol, phenol, chlorobutanol, benzalkonium chloride, and the like. In many cases, the formulation is stored at about 4°C. Formulations may also be lyophilized, in which case they generally include cryoprotectants such as sucrose, trehalose, lactose, maltose, mannitol, and the like. Lyophilized formulations can be stored over extended periods of time, even at ambient temperatures.
[0100] As such, administration of the compounds as described herein can be achieved in various ways, including oral, buccal, rectal, parenteral, intraperitoneal, intradermal, subcutaneous, intramuscular, transdermal, intratracheal, etc., administration. In some embodiments, administration is by bolus injection, e.g., subcutaneous bolus injection, intramuscular bolus injection, and the like.
[0101] The pharmaceutical compositions of the embodiments can be administered orally, parenterally or via an implanted reservoir. Oral administration or administration by injection is preferred.
[0102] Subcutaneous administration of a pharmaceutical composition of the embodiments is accomplished using standard methods and devices, e.g., needle and syringe, a subcutaneous injection port delivery system, and the like. See, e.g., U.S. Patent
Nos. 3,547,119; 4,755,173; 4,531,937; 4,311,137; and 6,017,328. A combination of a subcutaneous injection port and a device for administration of a pharmaceutical composition of the embodiments to a patient through the port is referred to herein as "a subcutaneous injection port delivery system." In many embodiments, subcutaneous administration is achieved by bolus delivery by needle and syringe.
[0103] In pharmaceutical dosage forms, the compounds as described herein may be administered in the form of their pharmaceutically acceptable salts, or they may also be used alone or in appropriate association, as well as in combination, with other pharmaceutically active compounds. The following methods and excipients are merely exemplary and are in no way limiting.
[0104] For oral preparations, the compounds as described herein can be used alone or in combination with appropriate additives to make tablets, powders, granules or capsules, for example, with conventional additives, such as lactose, mannitol, corn starch or potato starch; with binders, such as crystalline cellulose, cellulose derivatives, acacia, corn starch or gelatins; with disintegrators, such as corn starch, potato starch or sodium carboxymethylcellulose; with lubricants, such as talc or magnesium stearate; and if desired, with diluents, buffering agents, moistening agents, preservatives and flavoring agents.
[0105] The compounds as described herein can be formulated into preparations for injection by dissolving, suspending or emulsifying them in an aqueous or nonaqueous solvent, such as vegetable or other similar oils, synthetic aliphatic acid glycerides, esters of higher aliphatic acids or propylene glycol; and if desired, with conventional additives such as solubilizers, isotonic agents, suspending agents, emulsifying agents, stabilizers and preservatives.
[0106] Furthermore, the compounds as described herein can be made into suppositories by mixing with a variety of bases such as emulsifying bases or water-soluble bases. The compounds of the embodiments can be administered rectally via a suppository. The suppository can include vehicles such as cocoa butter, carbowaxes and polyethylene glycols, which melt at body temperature, yet are solidified at room temperature.
[0107] Unit dosage forms for oral or rectal administration such as syrups, elixirs, and suspensions may be provided wherein each dosage unit, for example, teaspoonful, tablespoonful, tablet or suppository, contains a predetermined amount of the
composition containing one or more compounds as described herein. Similarly, unit dosage forms for injection or intravenous administration may comprise the compounds as described herein in a composition as a solution in sterile water, normal saline or another pharmaceutically acceptable carrier.
[0108] The term "unit dosage form," as used herein, refers to physically discrete units suitable as unitary dosages for human and animal subjects, each unit containing a predetermined quantity of compounds of the embodiments calculated in an amount sufficient to produce the desired effect in association with a pharmaceutically acceptable diluent, carrier or vehicle. The specifications for the novel unit dosage forms of the embodiments depend on the particular compound employed and the effect to be achieved, and the pharmacodynamics associated with each compound in the host.
[0109] The pharmaceutically acceptable excipients, such as vehicles, adjuvants, carriers or diluents, are readily known in the art. Moreover, pharmaceutically acceptable auxiliary substances, such as pH adjusting and buffering agents, tonicity adjusting agents, stabilizers, wetting agents and the like, are readily known in the art. Treating a hepatitis virus infection
[0110] The methods and compositions described herein are generally useful in treatment of an of HCV infection.
[0111] Preferred embodiments provide a method of treating a hepatitis C virus infection in an individual, the method comprising administering to the individual an effective amount of a composition comprising a subject compound.
[0112] Preferred embodiments provide a method of treating liver fibrosis in an individual, the method comprising administering to the individual an effective amount of a composition comprising a subject compound.
[0113] Preferred embodiments provide a method of increasing liver function in an individual having a hepatitis C virus infection, the method comprising administering to the individual an effective amount of a composition comprising a subject compound.
[0114] Whether a subject method is effective in treating an HCV infection can be determined by a reduction in viral load, a reduction in time to seroconversion (virus undetectable in patient serum), an increase in the rate of sustained viral response to therapy, a reduction of morbidity or mortality in clinical outcomes, or other indicator of disease response.
[0115] In general, an effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, is an amount that is effective to reduce viral load or achieve a sustained viral response to therapy.
[0116] Whether a subject method is effective in treating an HCV infection can be determined by measuring viral load, or by measuring a parameter associated with HCV infection, including, but not limited to, liver fibrosis, elevations in serum transaminase levels, and necroinflammatory activity in the liver. Indicators of liver fibrosis are discussed in detail below.
[0117] In some embodiments, the methods involve administering an effective amount of a compound of Formulas XV, XVI, XVII or XVIII, optionally in combination with an effective amount of one or more additional antiviral agents. In some embodiments, an effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, is an amount that is effective to reduce viral titers to undetectable levels, e.g., to about 1000 to about 5000, to about 500 to about 1000, or to about 100 to about 500 genome copies/mL serum. In some embodiments, an effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, is an amount that is effective to reduce viral load to lower than 100 genome copies/mL serum.
[0118] In some embodiments, an effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, is an amount that is effective to achieve a 1.5-log, a 2-log, a 2.5-log, a 3-log, a 3.5-log, a 4-log, a 4.5-log, or a 5-log reduction in viral titer in the serum of the individual.
[0119] In many embodiments, an effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, is an amount that is effective to achieve a sustained viral response, e.g., non- detectable or substantially non-detectable HCV R A (e.g., less than about 500, less than about 400, less than about 200, or less than about 100 genome copies per milliliter serum) is found in the patient's serum for a period of at least about one month, at least about two months, at least about three months, at least about four months, at least about five months, or at least about six months following cessation of therapy.
[0120] As noted above, whether a subject method is effective in treating an HCV infection can be determined by measuring a parameter associated with HCV infection, such as liver fibrosis. Methods of determining the extent of liver fibrosis are
discussed in detail below. In some embodiments, the level of a serum marker of liver fibrosis indicates the degree of liver fibrosis.
[0121] As one non-limiting example, levels of serum alanine aminotransferase (ALT) are measured, using standard assays. In general, an ALT level of less than about 45 international units is considered normal. In some embodiments, an effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, is an amount effective to reduce ALT levels to less than about 45 IU/mL serum.
[0122] A therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, is an amount that is effective to reduce a serum level of a marker of liver fibrosis by at least about 10%, at least about 20%, at least about 25%, at least about 30%, at least about 35%, at least about 40%), at least about 45%, at least about 50%>, at least about 55%, at least about 60%>, at least about 65%, at least about 70%>, at least about 75%, or at least about 80%, or more, compared to the level of the marker in an untreated individual, or to a placebo-treated individual. Methods of measuring serum markers include immunological-based methods, e.g., enzyme-linked immunosorbent assays (ELISA), radioimmunoassays, and the like, using antibody specific for a given serum marker.
[0123] In many embodiments, an effective amount of a compound of Formulas XV, XVI, XVII or XVIII and an additional antiviral agent is a synergistic amount. As used herein, a "synergistic combination" or a "synergistic amount" of a compound of Formulas XV, XVI, XVII or XVIII and an additional antiviral agent is a combined dosage that is more effective in the therapeutic or prophylactic treatment of an HCV infection than the incremental improvement in treatment outcome that could be predicted or expected from a merely additive combination of (i) the therapeutic or prophylactic benefit of the compound of Formulas XV, XVI, XVII or XVIII when administered at that same dosage as a monotherapy and (ii) the therapeutic or prophylactic benefit of the additional antiviral agent when administered at the same dosage as a monotherapy.
[0124] In some embodiments, a selected amount of a compound of Formulas XV, XVI, XVII or XVIII and a selected amount of an additional antiviral agent are effective when used in combination therapy for a disease, but the selected amount of the compound of Formulas XV, XVI, XVII or XVIII and/or the selected amount of the
additional antiviral agent is less effective when used in monotherapy for the disease. Thus, the embodiments encompass (1) regimens in which a selected amount of the additional antiviral agent enhances the therapeutic benefit of a selected amount of the compound of Formulas XV, XVI, XVII or XVIII when used in combination therapy for a disease, where the selected amount of the additional antiviral agent provides negligible therapeutic benefit when used in monotherapy for the disease (2) regimens in which a selected amount of the compound of Formulas XV, XVI, XVII or XVIII enhances the therapeutic benefit of a selected amount of the additional antiviral agent when used in combination therapy for a disease, where the selected amount of the compound of Formulas XV, XVI, XVII or XVIII provides negligible therapeutic benefit when used in monotherapy for the disease and (3) regimens in which a selected amount of the compound of Formulas XV, XVI, XVII or XVIII and a selected amount of the additional antiviral agent provide a therapeutic benefit when used in combination therapy for a disease, where each of the selected amounts of the compound of Formulas XV, XVI, XVII or XVIII and the additional antiviral agent, respectively, provides negligible therapeutic benefit when used in monotherapy for the disease. As used herein, a "synergistically effective amount" of a compound of Formulas XV, XVI, XVII or XVIII and an additional antiviral agent, and its grammatical equivalents, shall be understood to include any regimen encompassed by any of (l)-(3) above.
Fibrosis
[0125] The embodiments provides methods for treating liver fibrosis (including forms of liver fibrosis resulting from, or associated with, HCV infection), generally involving administering a therapeutic amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents. Effective amounts of compounds of Formulas XV, XVI, XVII or XVIII, with and without one or more additional antiviral agents, as well as dosing regimens, are as discussed below.
[0126] Whether treatment with a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, is effective in reducing liver fibrosis can be determined by any of a number of well-established techniques for measuring liver fibrosis and liver function. Liver fibrosis reduction is determined by analyzing a liver biopsy sample. An analysis of a liver biopsy comprises assessments of two major components: necroinflammation assessed by "grade" as a measure of the
severity and ongoing disease activity, and the lesions of fibrosis and parenchymal or vascular remodeling as assessed by "stage" as being reflective of long-term disease progression. See, e.g., Brunt (2000) Hepatol. 31 :241-246; and METAVIR (1994) Hepatology 20: 15-20. Based on analysis of the liver biopsy, a score is assigned. A number of standardized scoring systems exist which provide a quantitative assessment of the degree and severity of fibrosis. These include the METAVIR, Knodell, Scheuer, Ludwig, and Ishak scoring systems.
[0127] The METAVIR scoring system is based on an analysis of various features of a liver biopsy, including fibrosis (portal fibrosis, centrilobular fibrosis, and cirrhosis); necrosis (piecemeal and lobular necrosis, acidophilic retraction, and ballooning degeneration); inflammation (portal tract inflammation, portal lymphoid aggregates, and distribution of portal inflammation); bile duct changes; and the Knodell index (scores of periportal necrosis, lobular necrosis, portal inflammation, fibrosis, and overall disease activity). The definitions of each stage in the METAVIR system are as follows: score: 0, no fibrosis; score: 1, stellate enlargement of portal tract but without septa formation; score: 2, enlargement of portal tract with rare septa formation; score: 3, numerous septa without cirrhosis; and score: 4, cirrhosis.
[0128] Knodell's scoring system, also called the Hepatitis Activity Index, classifies specimens based on scores in four categories of histologic features: I. Periportal and/or bridging necrosis; II. Intralobular degeneration and focal necrosis; III. Portal inflammation; and IV. Fibrosis. In the Knodell staging system, scores are as follows: score: 0, no fibrosis; score: 1, mild fibrosis (fibrous portal expansion); score: 2, moderate fibrosis; score: 3, severe fibrosis (bridging fibrosis); and score: 4, cirrhosis. The higher the score, the more severe the liver tissue damage. Knodell (1981) Hepatol. 1 :431.
[0129] The Scheuer scoring system scores are as follows: score: 0, no fibrosis; score: 1, enlarged, fibrotic portal tracts; score: 2, periportal or portal-portal septa, but intact architecture; score: 3, fibrosis with architectural distortion, but no obvious cirrhosis; score: 4, probable or definite cirrhosis. Scheuer (1991) J. Hepatol. 13:372.
[0130] The Ishak scoring system is described in Ishak (1995) J. Hepatol. 22:696-699. Stage 0, No fibrosis; Stage 1, Fibrous expansion of some portal areas, with or without short fibrous septa; stage 2, Fibrous expansion of most portal areas, with or without short fibrous septa; stage 3, Fibrous expansion of most portal areas with occasional portal to portal (P-P) bridging; stage 4, Fibrous expansion of portal areas with
marked bridging (P-P) as well as portal-central (P-C); stage 5, Marked bridging (P-P and/or P-C) with occasional nodules (incomplete cirrhosis); stage 6, Cirrhosis, probable or definite.
[0131] The benefit of anti-fibrotic therapy can also be measured and assessed by using the Child-Pugh scoring system which comprises a multicomponent point system based upon abnormalities in serum bilirubin level, serum albumin level, prothrombin time, the presence and severity of ascites, and the presence and severity of encephalopathy. Based upon the presence and severity of abnormality of these parameters, patients may be placed in one of three categories of increasing severity of clinical disease: A, B, or C.
[0132] In some embodiments, a therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, is an amount that effects a change of one unit or more in the fibrosis stage based on pre- and post-therapy liver biopsies. In particular embodiments, a therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, reduces liver fibrosis by at least one unit in the METAVIR, the Knodell, the Scheuer, the Ludwig, or the Ishak scoring system.
[0133] Secondary, or indirect, indices of liver function can also be used to evaluate the efficacy of treatment with a compound of Formulas XV, XVI, XVII or XVIII. Morphometric computerized semi- automated assessment of the quantitative degree of liver fibrosis based upon specific staining of collagen and/or serum markers of liver fibrosis can also be measured as an indication of the efficacy of a subject treatment method. Secondary indices of liver function include, but are not limited to, serum transaminase levels, prothrombin time, bilirubin, platelet count, portal pressure, albumin level, and assessment of the Child-Pugh score.
[0134] An effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, is an amount that is effective to increase an index of liver function by at least about 10%, at least about 20%, at least about 25%, at least about 30%, at least about 35%, at least about 40%, at least about 45%), at least about 50%>, at least about 55%, at least about 60%>, at least about 65%, at least about 70%>, at least about 75%, or at least about 80%>, or more, compared to the index of liver function in an untreated individual, or to a placebo-treated individual.
Those skilled in the art can readily measure such indices of liver function, using standard assay methods, many of which are commercially available, and are used routinely in clinical settings.
[0135] Serum markers of liver fibrosis can also be measured as an indication of the efficacy of a subject treatment method. Serum markers of liver fibrosis include, but are not limited to, hyaluronate, N-terminal procollagen III peptide, 7S domain of type IV collagen, C-terminal procollagen I peptide, and laminin. Additional biochemical markers of liver fibrosis include α-2-macroglobulin, haptoglobin, gamma globulin, apolipoprotein A, and gamma glutamyl transpeptidase.
[0136] A therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, is an amount that is effective to reduce a serum level of a marker of liver fibrosis by at least about 10%, at least about 20%, at least about 25%, at least about 30%, at least about 35%, at least about 40%), at least about 45%, at least about 50%>, at least about 55%, at least about 60%>, at least about 65%, at least about 70%>, at least about 75%, or at least about 80%, or more, compared to the level of the marker in an untreated individual, or to a placebo-treated individual. Those skilled in the art can readily measure such serum markers of liver fibrosis, using standard assay methods, many of which are commercially available, and are used routinely in clinical settings. Methods of measuring serum markers include immunological-based methods, e.g., enzyme-linked immunosorbent assays (ELISA), radioimmunoassays, and the like, using antibody specific for a given serum marker.
[0137] As used herein, a "complication associated with cirrhosis of the liver" refers to a disorder that is a sequellae of decompensated liver disease, i.e., or occurs subsequently to and as a result of development of liver fibrosis, and includes, but it not limited to, development of ascites, variceal bleeding, portal hypertension, jaundice, progressive liver insufficiency, encephalopathy, hepatocellular carcinoma, liver failure requiring liver transplantation, and liver-related mortality.
[0138] A therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, is an amount that is effective in reducing the incidence (e.g., the likelihood that an individual will develop) of a disorder associated with cirrhosis of the liver by at least about 10%, at least about 20%), at least about 25%, at least about 30%, at least about 35%, at least about 40%, at least about 45%, at least about 50%, at least about 55%, at least about 60%, at least
about 65%, at least about 70%, at least about 75%, or at least about 80%, or more, compared to an untreated individual, or to a placebo-treated individual.
[0139] Whether treatment with a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, is effective in reducing the incidence of a disorder associated with cirrhosis of the liver can readily be determined by those skilled in the art.
[0140] Reduction in liver fibrosis can increase liver function. Thus, the embodiments provide methods for increasing liver function, generally involving administering a therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents. Liver functions include, but are not limited to, synthesis of proteins such as serum proteins (e.g., albumin, clotting factors, alkaline phosphatase, aminotransferases (e.g., alanine transaminase, aspartate transaminase), 5 '-nucleosidase, γ-glutaminyltranspeptidase, etc.), synthesis of bilirubin, synthesis of cholesterol, and synthesis of bile acids; a liver metabolic function, including, but not limited to, carbohydrate metabolism, amino acid and ammonia metabolism, hormone metabolism, and lipid metabolism; detoxification of exogenous drugs; a hemodynamic function, including splanchnic and portal hemodynamics; and the like.
[0141] Whether a liver function is increased is readily ascertainable by those skilled in the art, using well-established tests of liver function. Thus, synthesis of markers of liver function such as albumin, alkaline phosphatase, alanine transaminase, aspartate transaminase, bilirubin, and the like, can be assessed by measuring the level of these markers in the serum, using standard immunological and enzymatic assays. Splanchnic circulation and portal hemodynamics can be measured by portal wedge pressure and/or resistance using standard methods. Metabolic functions can be measured by measuring the level of ammonia in the serum.
[0142] Whether serum proteins normally secreted by the liver are in the normal range can be determined by measuring the levels of such proteins, using standard immunological and enzymatic assays. Those skilled in the art know the normal ranges for such serum proteins. The following are non-limiting examples. The normal level of alanine transaminase is about 45 IU per milliliter of serum. The normal range of aspartate transaminase is from about 5 to about 40 units per liter of serum. Bilirubin is measured using standard assays. Normal bilirubin levels are usually less than about 1.2
mg/dL. Serum albumin levels are measured using standard assays. Normal levels of serum albumin are in the range of from about 35 to about 55 g/L. Prolongation of prothrombin time is measured using standard assays. Normal prothrombin time is less than about 4 seconds longer than control.
[0143] A therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, is one that is effective to increase liver function by at least about 10%, at least about 20%, at least about 30%), at least about 40%>, at least about 50%>, at least about 60%>, at least about 70%>, at least about 80%, or more. For example, a therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, is an amount effective to reduce an elevated level of a serum marker of liver function by at least about 10%, at least about 20%, at least about 30%, at least about 40%), at least about 50%>, at least about 60%>, at least about 70%>, at least about 80%>, or more, or to reduce the level of the serum marker of liver function to within a normal range. A therapeutically effective amount of a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents, is also an amount effective to increase a reduced level of a serum marker of liver function by at least about 10%, at least about 20%, at least about 30%, at least about 40%, at least about 50%, at least about 60%), at least about 70%>, at least about 80%>, or more, or to increase the level of the serum marker of liver function to within a normal range.
Dosages, Formulations, and Routes of Administration
[0144] In the subject methods, the active agent(s) (e.g., compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agents) may be administered to the host using any convenient means capable of resulting in the desired therapeutic effect. Thus, the agent can be incorporated into a variety of formulations for therapeutic administration. More particularly, the agents of the embodiments can be formulated into pharmaceutical compositions by combination with appropriate, pharmaceutically acceptable carriers or diluents, and may be formulated into preparations in solid, semi-solid, liquid or gaseous forms, such as tablets, capsules, powders, granules, ointments, solutions, suppositories, injections, inhalants and aerosols.
Other antiviral or antifibrotic agents
[0145] As discussed above, a subject method will in some embodiments be carried out by administering a compound of Formulas XV, XVI, XVII or XVIII, and optionally one or more additional antiviral agent(s).
[0146] In some embodiments, the method further includes administration of one or more interferon receptor agonist(s)
[0147] In other embodiments, the method further includes administration of pirfenidone or a pirfenidone analog.
[0148] Additional antiviral agents that are suitable for use in combination therapy include, but are not limited to, nucleotide and nucleoside analogs. Non-limiting examples include azidothymidine (AZT) (zidovudine), and analogs and derivatives thereof; 2',3 '-dideoxyinosine (DDI) (didanosine), and analogs and derivatives thereof; 2',3 '-dideoxycytidine (DDC) (dideoxycytidine), and analogs and derivatives thereof; 2',3 '-didehydro-2',3 '-dideoxythymidine (D4T) (stavudine), and analogs and derivatives thereof; combivir; abacavir; adefovir dipoxil; cidofovir; ribavirin; ribavirin analogs; and the like.
[0149] In some embodiments, the method further includes administration of ribavirin. Ribavirin, l- -D-ribofuranosyl-lH-l ,2,4-triazole-3-carboxamide, available from ICN Pharmaceuticals, Inc., Costa Mesa, Calif, is described in the Merck Index, compound No. 8199, Eleventh Edition. Its manufacture and formulation is described in U.S. Pat. No. 4,21 1 ,771. Some embodiments also involve use of derivatives of ribavirin (see, e.g. , U.S. Pat. No. 6,277,830). The ribavirin may be administered orally in capsule or tablet form, or in the same or different administration form and in the same or different route as the subject compound. Of course, other types of administration of both medicaments, as they become available are contemplated, such as by nasal spray, transdermally, intravenously, by suppository, by sustained release dosage form, etc. Any form of administration will work so long as the proper dosages are delivered without destroying the active ingredient.
[0150] In some embodiments, the method further includes administration of ritonavir. Ritonavir, 10-hydroxy-2-methyl-5 -( 1 -methy lethyl)- 1 - [2-( 1 -methylethyl)-4- thiazolyl]-3,6-dioxo-8,l l-bis(phenylmethyl)-2,4,7,12-tetraazatridecan-13-oic acid, 5- thiazolylmethyl ester [5i -(5i?*,8i?*,10i?*,l li?*)], available from Abbott Laboratories, is an inhibitor of the protease of the human immunodeficiency virus and also of the
cytochrome P450 3 A and P450 2D6 liver enzymes frequently involved in hepatic metabolism of therapeutic molecules in man.
[0151] In some embodiments, the method further includes administration of a protease inhibitor. In some embodiments, the method further includes administration of an NS5A inhibitor. In some embodiments, the method further includes administration of a helicase inhibitor. In some embodiments, the method further includes administration of a polymerase inhibitor.
[0152] In some embodiments, an additional antiviral agent is administered during the entire course of the subject compound treatment. In other embodiments, an additional antiviral agent is administered for a period of time that is overlapping with that of the subject compound treatment, e.g., the additional antiviral agent treatment can begin before the subject compound treatment begins and end before the subject compound treatment ends; the additional antiviral agent treatment can begin after the subject compound treatment begins and end after the subject compound treatment ends; the additional antiviral agent treatment can begin after the subject compound treatment begins and end before the subject compound treatment ends; or the additional antiviral agent treatment can begin before the subject compound treatment begins and end after the subject compound treatment ends.
Methods of Treatment
Monotherapies
[0153] The compounds as described herein may be used in acute or chronic therapy for HCV disease. In many embodiments, the compounds as described herein can be administered for a period of about 1 day to about 7 days, or about 1 week to about 2 weeks, or about 2 weeks to about 3 weeks, or about 3 weeks to about 4 weeks, or about 1 month to about 2 months, or about 3 months to about 4 months, or about 4 months to about 6 months, or about 6 months to about 8 months, or about 8 months to about 12 months, or at least one year, and may be administered over longer periods of time. The compounds as described herein can be administered 5 times per day, 4 times per day, tid, bid, qd, qod, biw, tiw, qw, qow, three times per month, or once monthly. In other embodiments, the NS5A inhibitor compound is administered as a continuous infusion.
[0154] In many embodiments, an compounds as described herein of the embodiments can be administered orally.
[0155] In connection with the above-described methods for the treatment of HCV disease in a patient, a compound as described herein may be administered to the patient at a dosage from about 0.01 mg to about 100 mg/kg patient bodyweight per day, in 1 to 5 divided doses per day. In some embodiments, the compounds as described herein can be administered at a dosage of about 0.5 mg to about 75 mg/kg patient bodyweight per day, in 1 to 5 divided doses per day.
[0156] The amount of active ingredient that may be combined with carrier materials to produce a dosage form can vary depending on the host to be treated and the particular mode of administration. A typical pharmaceutical preparation can contain from about 5% to about 95% active ingredient (w/w). In other embodiments, the pharmaceutical preparation can contain from about 20% to about 80% active ingredient.
[0157] Those of skill will readily appreciate that dose levels can vary as a function of the specific compound, the severity of the symptoms and the susceptibility of the subject to side effects. Preferred dosages for a given compound as described herein can be readily determinable by those of skill in the art by a variety of means. A preferred means can be to measure the physiological potency of a given interferon receptor agonist.
[0158] In many embodiments, multiple doses of NS5A inhibitor compound are administered. For example, an NS5A inhibitor compound is administered once per month, twice per month, three times per month, every other week (qow), once per week (qw), twice per week (biw), three times per week (tiw), four times per week, five times per week, six times per week, every other day (qod), daily (qd), twice a day (qid), or three times a day (tid), over a period of time ranging from about one day to about one week, from about two weeks to about four weeks, from about one month to about two months, from about two months to about four months, from about four months to about six months, from about six months to about eight months, from about eight months to about 1 year, from about 1 year to about 2 years, or from about 2 years to about 4 years, or more. Combination therapies with a TNF-q antagonist and an interferon
[0159] Some embodiments provide a method of treating an HCV infection in an individual having an HCV infection, the method comprising administering an effective amount of one of the compounds as decribed herein, and effective amount of a TNF-a antagonist, and an effective amount of one or more interferons.
Subjects Suitable for Treatment
[0160] In certain embodiments, the specific regimen of drug therapy used in treatment of the HCV patient is selected according to certain disease parameters exhibited by the patient, such as the initial viral load, genotype of the HCV infection in the patient, liver histology and/or stage of liver fibrosis in the patient.
[0161] Any of the above treatment regimens can be administered to individuals who have been diagnosed with an HCV infection. Any of the above treatment regimens can be administered to individuals having advanced or severe stage liver fibrosis as measured by a Knodell score of 3 or 4 or no or early stage liver fibrosis as measured by a Knodell score of 0, 1, or 2. Any of the above treatment regimens can be administered to individuals who have failed previous treatment for HCV infection ("treatment failure patients," including non-responders and relapsers).
[0162] Individuals who have been clinically diagnosed as infected with HCV are of particular interest in many embodiments. Individuals who are infected with HCV are identified as having HCV R A in their blood, and/or having anti-HCV antibody in their serum. Such individuals include anti-HCV ELISA-positive individuals, and individuals with a positive recombinant immunoblot assay (RIB A). Such individuals may also, but need not, have elevated serum ALT levels.
[0163] Individuals who are clinically diagnosed as infected with HCV include na'ive individuals (e.g., individuals not previously treated for HCV, particularly those who have not previously received IFN-a-based and/or ribavirin-based therapy) and individuals who have failed prior treatment for HCV ("treatment failure" patients). Treatment failure patients include non-responders (i.e., individuals in whom the HCV titer was not significantly or sufficiently reduced by a previous treatment for HCV, e.g., a previous IFN-a monotherapy, a previous IFN-a and ribavirin combination therapy, or a previous pegylated IFN-a and ribavirin combination therapy); and relapsers (i.e., individuals who were previously treated for HCV, e.g., who received a previous IFN-a monotherapy, a previous IFN-a and ribavirin combination therapy, or a previous pegylated IFN-a and ribavirin combination therapy, whose HCV titer decreased, and subsequently increased).
[0164] In particular embodiments of interest, individuals have an HCV titer of at least about 105, at least about 5 x 105, or at least about 106, or at least about 2 x 106, genome copies of HCV per milliliter of serum. The patient may be infected with any HCV genotype (genotype 1, including la and lb, 2, 3, 4, 6, etc. and subtypes (e.g., 2a, 2b,
3a, etc.)), particularly a difficult to treat genotype such as HCV genotype 1 and particular HCV subtypes and quasispecies.
[0165] Also of interest are HCV -positive individuals (as described above) who exhibit severe fibrosis or early cirrhosis (non-decompensated, Child' s-Pugh class A or less), or more advanced cirrhosis (decompensated, Child' s-Pugh class B or C) due to chronic HCV infection and who are viremic despite prior anti-viral treatment with IFN-a- based therapies or who cannot tolerate IFN-a-based therapies, or who have a contraindication to such therapies. In particular embodiments of interest, HCV-positive individuals with stage 3 or 4 liver fibrosis according to the METAVIR scoring system are suitable for treatment with the methods described herein. In other embodiments, individuals suitable for treatment with the methods of the embodiments are patients with decompensated cirrhosis with clinical manifestations, including patients with far- advanced liver cirrhosis, including those awaiting liver transplantation. In still other embodiments, individuals suitable for treatment with the methods described herein include patients with milder degrees of fibrosis including those with early fibrosis (stages 1 and 2 in the METAVIR, Ludwig, and Scheuer scoring systems; or stages 1, 2, or 3 in the Ishak scoring system.).
Synthesis
[0166] The compounds and processes of the present disclosure will be better understood in connection with the following synthetic schemes which illustrate the methods by which the compounds of the present disclosure may be prepared. Starting materials can be obtained from commercial sources or prepared by well-established literature methods known to those of ordinary skill in the art. The variables are as defined above unless otherwise noted below.
SECTION I
PREPARATION OF SYNTHETIC INTERMEDIATE: SECTION I
Example X-IV: Preparation of Intermediate I-IVc
-IV
HATU, DIEA, DCM
l-IVa
General Procedure X-W
[0167] To a mixture of 2-methyl-L-proline (1.0 g, 7.8 mmol) in 20 mL of dry methanol was added SOCl2 (2.8 g, 23.3 mmol) dropwise at 0°C under nitrogen protection.
The resulting mixture was stirred at room temperature overnight, and then the solvent was removed under reduced pressure to afford compound I-IVa as an HCI salt (1.4 g, yield
100%). 1H NMR (300 MHz, CD3OD): δ 3.86 (s, 3H), 3.42-3.46 (m, 2H), 2.36-2.45 (m,
1H), 2.00-2.19 (m, 3H), 1.68 (s, 3H).
Scheme X-IVb
[0168] To a solution of compound I-IVa (1.35 g, 7.7 mmol) in 30 mL of
DCM was added compound Vl-IIa (1.5 g, 8.5 mmol), HATU (4.4 g, 11.6 mmol) and
DIEA (3 g, 23 mmol). The resulting mixture was stirred at room temperature overnight.
Subsequently, the mixture was diluted with DCM and washed with brine. The organic layers were dried over anhydrous Na2S04 and concentrated. The residue was purified by column chromatography (PE/EA=3/1) to afford compound I-IVb (1.5 g, yield 65%). MS
(ESI) m/z (M+H)+ 301.
Scheme X-IVc
General Procedure X-Y
[0169] A mixture of compound I-IVb (1.5 g, 5 mmol) and NaOH (0.6 g, 15 mmol) in MeOH (30 mL) and H20 (5 mL) was stirred at 70°C for 2 hours. The methanol under reduce pressure and the residue was dissolved with 20 mL of H20, then the solution was acidfied to pH 2-3 with 2N HC1 and extracted with DCM (50 mLx2). The organic layer was washed with brine, dried over anhydrous Na2S04 and concentrated to afford compound I-IVc (0.8 g, yield 57%), which was used in next step without further purification. 1H NMR (300 MHz, DMSO-d6): δ 12.20 (s, 1H), 7.24 (d, J=8.4 Hz, 1 H),
3.54-3.98 (m, 3H), 3.50 (s, 3H), 1.78-2.05 (m, 5H), 1.34 (s, 3H), 0.84-0.88 (m, 6H).
SECTION II
PREPARATION OF COMPOUNDS: SECTION II
Example XI-I: General synthetic protocol for the preparation of compounds 701-705
-I
IX-llla Xl-la
General Procedure XI- A
[0170] A mixture of 2-bromo-l-(4-bromophenyl)ethanone (IX-IIIa) (8 g, 29 mmol) and N-acetylguanidine (5.84 g, 58 mmol) in DMF (80 mL) was stirred at r.t. for 48 hrs. Then the mixture was poured into water, and extracted with EtOAc. The organic layer was separated, washed with water and brine, dried over MgS04, filtered and concentrated. The residue was purified by column chromatography (PE:EA= 10: 1-3: 1) to afford compound Xl-la (2.5 g, yield 31%). MS (ESI) m/z (M+H)+ 281.
Scheme XI-Ib
Xl-la Xl-lb
General Procedure XI-B
[0171] Cone. H2SO4 (3 mL) was added to a solution of compound Xl-la (2.5 g, 8.9 mmol) in MeOH (10 mL) and H20 (10 mL). The reaction mixture was stirred at reflux for 5 hrs. After being cooled to r.t., the mixture was basified with Na2C03 to pH~9, and extracted with DCM. The organic layer was separated, dried over MgS04, filtered and concentrated to afford compound XI-Ib (1.4 g, yield 65%). MS (ESI) m/z
(M+H)+ 239.
Scheme XI-Ic
General Procedure XI-C
[0172] Compound XI-Ib (1 eq.) was added to a mixture of acid XI-Ic (1.2 eq.), HATU (1.2 eq.) and DIEA (3 eq.) in DCM. The reaction mixture was stirred at r.t. for 1 h, and then diluted with DCM, washed with water and brine. The organic layer was separated, dried, filtered and concentrated under reduced pressure. The residue was purified by Prep-TLC to afford a compound of general structure Xl-ld. -ld
General Procedure XI-D
[0173] To a stirred mixture of a compound of general structure Xl-Id (1 eq.), compound I-XVIIaa (1.1 eq.), and CS2CO3 (2.2 eq.) in 1,4-dioxane and H20 (v/v=7/l) was added Pd(dppf)Cl2 (10 mol%) under N2 protection. The reaction mixture was stirred at 90 °C for 12hrs. After being cooled to r.t., the mixture was diluted with EtOAc, washed with water and brine. The organic layer was separated, dried over MgSC^, filtered and concentrated under reduced pressure. The residue was purified by Prep-TLC (PE:EA=1 :2) to afford a compound of general structure Xl-Ie. -Ie
General Procedure XI-E
[0174] A compound of general structure Xl-Ie (1 eq.) was dissolved in a solution of TFA in DCM (v%=30%, 2 mL). The reaction mixture was stirred at r.t. for 1 h. After concentrated under reduced pressure, the residue was neutralized with aq. NaOH
(1 M), and extracted with DCM. The organic layer was dried over MgSC^, filtered and concentrated to afford a compound of general structure ΧΙ-If, which was used directly in the next reaction without further purification. -If
General Procedure XI-F
[0175] To a stirred mixture of compound VII-IIA (1.5 eq.), HATU (1.5 eq.) and DIEA (3 eq.) in DCM was added a compound of general structure Xl-If (1 eq.). The reaction mixture was stirred at r.t. for 3 hrs. After diluted with DCM, the organic layer was washed with water and brine, filtered and concentrated under reduced pressure. The residue was purified by Prep-HPLC to afford a compound of general structure Xl-Ig.
[0176] The above synthetic approach was adopted for preparation of the following compounds:
Example XI-Ia: Synthesis of Compound 701
[0177] Compound 701 was prepared according to General Procedure XI-A-F, 15 mg, yield 63%; 1H NMR (CD3OD, 400MHz) δ 7.80-7.64 (m, 8H), 7.32 (s, 1H), 7.24 (s, 1H), 5.19-5.21 (m, 1H), 4.25 (d, J=7. 6Hz, 1H), 3.88-4.03 (m, 2H), 3.67 (s, 3 H), 2.46 (t, J=7. 6Hz, 2H), 2.18-2.40 (m, 3H), 1.98-2.13 (m, 2H), 1.68-1.76 (m, 2H), 1.42-1.48 (m, 2H), 0.91-1.02 (m, 9H). MS (ESI) m/z (M+H)+ 612.4.
Example XI -lb: Synthesis of Compound 702
702
[0178] Compound 702 was prepared according to General Procedure XI-A-F, 10 mg, yield 18%; 1H NMR (MeOD, 400MHz) δ 7.85-7.64 (m, 8H), 7.35 (brs, 1H), 7.26 (s, 1H), 5.17-5.25 (m, 1H), 4.20-4.30 (m, 1H), 3.85-4.09 (m, 2H), 3.70 (s, 3 H), 2.46-2.48 (m, 2H), 2.24 (s, 3H), 1.98-2.14 (m, 3H), 0.94-1.02 (m, 6H). MS (ESI) m / z (M+H)+ 570.2.
Example XI -Ic: Synthesis of Compound 703
703
[0179] Compound 703 was prepared according to General Procedure XI-A-F, 10 mg, yield 19%; 1H NMR (MeOD, 400MHz) δ 7.66-7.78 (m, 8H), 7.33 (s, 1H), 7.25 (s, 1H), 5.18-5.21 (m, 1H), 4.25 (d, J=7.6 Hz, 1H), 3.87-4.02 (m, 2H), 3.67 (s, 3 H), 1.94- 2.44 (m, 12H), 0.91-0.98 (m, 6H). MS (ESI) m / z (M+H)+ 610.3.
Example XI-Id: Synthesis of Compound 704
704
[0180] Compound 704 was prepared according to General Procedure XI-A-F, 9.4 mg, yield 18%; 1H NMR (CD3OD, 400MHz) δ 7.63-7.75 (m, 8H), 7.30 (s, 1H), 7.22 (s, 1H), 5.13-5.20 (m, 1H), 4.22 (d, J=7.5 Hz, 1H), 3.86-4.01 (m, 2H), 3.65 (s, 3 H), 2.85- 2.91 (m, 1H), 2.17-2.37 (m, 3H), 1.76-2.07 (m, 8H), 1.66-1.68 (m, 2H), 0.89-0.95 (m, 6H). MS (ESI) m / z (M+H)+ 624.4.
Example XI-Id: Synthesis of Compound 705
705
[0181] Compound 705 was prepared according to General Procedure XI-A-F, 10.5 mg, yield 15%; 1H NMR (CD3OD 400MHz) δ 7.61-7.80 (m, 8H), 7.32 (brs, 1H), 7.23 (s, 1H), 5.15-5.22 (m, 1H), 4.23 (d, J=7.6 Hz, 1H), 3.84-4.09 (m, 2H), 3.65 (s, 3 H), 2.18-2.48 (m, 4H), 2.05-2.09 (m, 2H), 1.83-1.93 (m, 4H), 1.72-1.75 (m, 1H), 1.51-1.57 (m, 2H), 1.30-1.40 (m, 3H), 0.99-0.89 (m, 6H). MS (ESI) m / z (M+H)+ 638.4.
Example XI-II: General synthetic protocol for the preparation of compounds 706-707
-II
General Procedure XI-G
[0182] A mixture of 2-bromo-l-(4-bromophenyl)ethanone (IX-IIIa) (20 g,
72.2 mmol), compound I-IIh (23 g, 84.5 mmol) and DIEA (24 g, 200 mmol) in THF (250
mL) was stirred at r.t. for 12 hrs. The mixture was concentrated in vacuo to remove THF and then the residue was re-dissolved in EtOAc. The mixture was washed with water and brine, dried over MgS04 and concentrated. The residue was purified by column chromatography (PE:EA=5: 1-1 : 1) to afford compound XI-IIa (25 g, yield 74%). MS (ESI) m/z (M+H)+ 470. -IIb
General Procedure XI-H
[0183] A mixture of compound XI-IIa (25 g, 53.1 mmol) and NH4OAc (40 g,
530 mmol) in toluene (250 mL) was stirred at reflux for 10 hrs. The mixture was diluted with EtOAc, washed with water and brine, dried over MgS04, filtered and concentrated.
The residue was purified by column chromatography (PE:EA=5: 1-1 :2) to afford compound XI-IIb (15.2 g, yield: 64%). MS (ESI) m/z (M+H)+ 450. -IIc
General Procedure XI-I
[0184] To a stirred mixture of compound XI-IIb (15.2 g, 33.9 mmol),
Bis(pinacolato)diboron (12.9 g, 50.8 mmol), and K2CO3 (9.5 g, 67.7 mmol) in 1,4- dioxane (150 mL) was added Pd(dppf)Cl2 (1.5 g) under N2 protection. The resulting mixture was stirred at 90 °C for 12 hrs. After cooling r.t., the mixture was diluted with
EtOAc, washed with water and brine, dried over MgS04 and concentrated. The residue was purified by column chromatography (PE:EA=1 :4~1 : 10) to afford compound XI-IIc
(7.2 mg, yield 43%). MS (ESI) m/z 497 (M+H)+.
-IId
General Procedure XI-J
[0185] To a stirred mixture of compound XI-IIc (2.2 g, 4.4 mmol), compound
Xl-Ib (1.0 g, 4.2 mmol) and Cs2C03 (2.96 g, 8.4 mmol) in 1,4-dioxane (30 mL) and H20
(3 mL) was added Pd(dppf)Cl2 (200 mg) under N2 protection. The reaction mixture was stirred at 90 °C overnight. The mixture was then diluted with EtOAc, washed with water and brine, dried over MgS04 and concentrated. The residue was purified by column chromatography (EA~EA:MeOH=20: l) to afford compound XI-IId (500 mg, yield 23%).
MS (ESI) m/z (M+H)+ 528.
-IIe
General Procedure XI-K
[0186] A mixture of compound XI-IId (1 eq.), HATU (1.2 eq.), DIEA (2 eq.) and general compound XI-IIe (1.2 eq.) in DCM was stirred at r.t. for 12 hrs. The mixture was diluted with DCM, washed with water and brine, separated the organic layer, dried over Na2S04 and concentrated. The residue was purified by Prep-HPLC to afford general compound XI-IIf .
[0187] The above synthetic approach was adopted for preparation of the following compounds:
Example XI-IIa: Synthesis of Compound 706
706
[0188] Compound 706 was prepared according to General Procedures XI-G to XI-K, 10.2 mg, yield 17.3%; 1H NMR (CD3OD, 400MHz) δ 7 '.66-7.79 (m, 8H), 7.34 (s, 1H), 7.25 (s, 1H), 5.17-5.21 (m, 1H), 4.25 (d, J=7.6 Hz, 1H), 3.87-4.06 (m, 2H), 3.67 (s, 3 H), 2.44 (t, J=7.6 Hz, 2H), 2.17-2.39 (m, 3H), 1.95-2.13 (m, 2H), 1.73-1.82 (m, 2H), 0.91-1.07 (m, 9H). MS (ESI) m/z (M+H) 598.2.
Example XI -lib: Synthesis of Compound 707
707
[0189] Compound 707 was prepared according to General Procedures XI-G to XI-K, 20 mg, yield 12.8%; 1H NMR (CD3OD, 400MHz) δ 7 '.66-7 '.78 (m, 8H), 7.34 (s, 1H), 7.26 (s, 1H), 5.18-5.21 (m, 1H), 4.13-4.29 (m, 2H), 3.85-4.05 (m, 2H), 3.70 (s, 3H), 3.67 (s, 3 H), 1.96-2.44 (m, 6H), 0.96-1.07 (m, 12H). MS (ESI) m/z (M+H)+ 685.4.
Example XI -III: Preparation of Compound 708
-III
General Procedure XI-L
[0190] To a stirred solution of compound XI-IId (100 mg, 0.19 mmol) and
Et3N (30 mg, 0.3 mmol) in DCM (2 mL) was added propane- 1-sulfonyl chloride (42 mg,
0.3 mmol), and the resulting mixture was stirred at r.t. for 1 h. The mixture was diluted with DCM, washed with water and brine, and the organic layer was separated, dried and
concentrated. The residue was purified by Prep-HPLC to afford compound 708 (20 mg, yield 16.7%). 1H NMR (CD3OD, 400MHz) δ 7.64-7.80 (m, 8H), 7.33 (s, 2H), 5.17-5.21 (m, IH), 4.25 (d, J=7.2 Hz, IH), 3.84-4.05 (m, 2H), 3.67 (s, 3 H), 3.54-3.58 (m, 2H), 2.19-2.39 (m, 3H), 1.96-2.12 (m, 2H), 1.81-1.91 (m, 2H), 1.07 (t, J=7.6 Hz, 3H), 0.91- 0.97 (m, 6H). MS (ESI) m/z (M+H)+ 634.4.
Example XI -IV: Preparation of Compound 709
-IV
General Procedure XI-M
[0191] To a stirred mixture of compound Xl-Ib (2 g, 8.4 mmol), compound I-
XVIIaa (3.86 g, 8.8 mmol), and Cs2C03 (5.93 g, 16.8 mmol) in 1,4-dioxane (30 mL) and
H20 (2 mL) was added Pd(dppf)Cl2 (300 mg) under N2 protection. The resulting mixture was stirred at 100 °C for 16 hrs. The mixture was diluted with EtOAc, washed with water and brine, dried over MgS04 and concentrated. The residue was purified by column chromatography (PE:EA=2: l-EA:MeOH=15: l) to afford compound XI-IVa (900 mg, yield 23%). MS (ESI) m/z (M+H)+ 471.
-IVb
General Procedure XI-N
[0192] To a stirred solution of compound XI-IVa (100 mg, 0.21 mmol) in pyridine was added benzoyl chloride (42 mg, 0.3 mmol), the reaction mixture was stirred at r.t. for 2 hrs. The solvent was concentrated under vacuum, re-dissolved in EtOAc, washed with water and brine, dried over MgS04 and concentrated to afford compound
XI-IVb (60 mg, yield 49 %), which was used directly in the next reaction without further purification. MS (ESI) m/z (M+H)+ 575.
-IVc
[0193] To compound XI-IVb (60 mg, 0.1 mmol) in DCM was added TFA
(0.5 mL). The reaction mixture was stirred at r.t. for 1 h. The solvent was concentrated under vacuum to afford compound XI-IVc (40 mg, yield: 80.8 %), which was used directly in the next reaction without further purification. MS (ESI) m/z (M+H)+ 475.
-IVd
General Procedure XI-P
[0194] To a stirred mixture of compound XI-IVc (40 mg, 0.08 mmol), HATU
(46 mg, 0.12 mmol) and DIEA (21 mg, 0.16 mmol) in DCM was added compound VII-
IIA (21 mg, 0.12 mmol). The resulting mixture was stirred at r.t. for 1 h and then diluted with DCM, washed with water and brine. The organic layer was separated, dried and concentrated. The residue was purified by prep-HPLC to afford compound 709 (11 mg, yield 21%). 1H NMR (CD3OD, 400MHz) δ 7.64-7.83 (m, 9H), 7.14-7.53 (m, 6H), 5.31-
5.37 (m, 1H), 4.17 (d, J=6.8 Hz, 1H), 3.88-3.92 (m, 1H), 3.70 (s, 3H), 3.61-3.67 (m, 3 H),
1.95-2.49 (m, 5H), 1.02-1.07 (m, 6H). MS (ESI) m/z (M+H)+ 632.4.
Example XI-V: Preparation of Compound 710
-V
General Procedure XI-Q
[0195] Compound XI- Va (65 mg, 0. 3 mmol) was dissolved in DCM (2 mL), followed by addition of Compound XI-IIa (100 mg, 0.19 mmol), HATU (114 mg, 0. 3 mmol) and DIEA (52 mg, 0.4 mmol). The resulting mixture was stirred at r.t. for 12 hrs.
The mixture was then diluted with DCM (40 mL), ad washed with water and brine. The organic layer was separated, dried over sodium sulfate and concentrated in vacuo. The residue was purified by Prep-HPLC to afford Compound 710 (15 mg, yield 11%). *H NMR (400MHz, CD3OD) δ 7.66-7.81 (m, 8H), 7.34 (s, 1H), 7.27 (s, 1H), 5.18-5.23 (m, 1H), 4.25 (d, J=7.6 Hz, 1H), 4.10-4.15 (m, 1H), 3.98-4.06 (m, 1H), 3.86-3.94 (m, 1H), 3.67 (s, 3H), 2.02-2.41 (m, 6H), 1.49 (s, 9H), 1.07-0.91 (m, 12H). MS (ESI) m/z (M+H)+ 727.5.
Example XI -VI: Preparation of Compounds 711-713
-VI
R=Pr, 713
Scheme XI- Via Boc20
H2N NH2 ► Boc,
NaOH, Acetone, N Λ. NH2
HCI H20 H
General Procedure XI-R
[0196] Guanidine hydrochloride (200 g, 2.1 mol) was dissolved in acetone
(200 mL), followed by addition of a solution of aq. NaOH (10.5 M, 400 mL) and Di-tert- butyl dicarbonate (113 g, 0.52 mol). The reaction mixture was stirred at 0°C for 8 hrs.
The mixture was concentrated in vacuo to remove acetone, and then it was poured into
water (200 mL), and extracted with EtOAc (300 mL x 3). The combined organic layers were washed with brine, dried over MgS04 and concentrated under reduced pressure. The crude N-Boc-guanidine was used in next step directly without further purification (43 g, yield 52%). -VIb
General Procedure XI-R
[0197] A flask was charged with crude N-Boc-guanidine (23 g, 144 mmol) and DMF (50 mL) and then Nal (5 g, 33.3 mmol) and 4-bromophenacyl bromide (10 g,
36.1 mmol) were added thereto at 0°C. The resulting mixture was slowly warmed to r.t. and stirred for an additional 12 hrs. The mixture was poured into water (100 mL), and extracted with EtOAc (100 mL x 3). The combined organic layers were washed with brine, dried over MgS04 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (Petroleum ether: EtOAc =10: 1) to afford compound XI- Via (4.1 g, yield 33%). MS (ESI) m/z (M+H)+ 339. -VIc
Xl-Vla Xl-Vlba
General Procedure XI- S
[0198] To a solution of compound XI- Via (170 mg, 0.5 mmol) in anhydrous
THF (3 mL) was added NaHMDS (1 mL, 1 mmol) at 0°C. The mixture was stirred at 0°C for 10 min, and then a solution of methyl chloro formate (94 mg, 1 mmol) in THF (1 mL) was added, the reaction mixture was stirred at 0°C for 30 min. After the starting material was consumed, the mixture was poured into water (20 mL), and extracted with EtOAc (30 mL x 3). The combined organic layers were washed with brine, dried over MgS04 and concentrated under reduced pressure. The residue was purified by prep-TLC (Petroleum ether: EtOAc =3: 1) to afford compound XI-VIbb (100 mg, yield 44%). MS (ESI) m/z
(M+H)+ 455.
[0199] The following compounds were prepared using General Procedure XI-
S:
XI-VIba Xl-VIca
General Procedure XI-T
[0200] Compound XI-VIba (80 mg, 0.18 mmol) was taken up with MeOH (2 mL) and aq. KOH (1 M, 1 mL). The resulting mixture was stirred at r.t. for 5 hrs. Then the mixture was concentrated in vacuo to remove MeOH, and the residue was diluted with water (10 mL), and extracted with DCM (20 mL x 3). The organic layers were combined, washed with water and brine, dried over MgS04 and concentrated under reduced pressure. The residue was purified by prep-TLC (Petroleum ether: EtOAc =3: 1) to afford compound Xl-VIca (50 mg, yield 96%). MS (ESI) m/z (M+H)+ 297.
[0201] The following compounds were prepared using General Procedure XI-
Xl-Vlcb Xl-Vlcc
-VIe
Pd(dppf)CI2, K2C03,
dioxane/H20, 90°C
General Procedure XI-U
[0202] A flask was charged with compound Xl-VIca (50 mg, 0.17 mmol), compound XI-IIc (85 mg, 0.17 mmol), K2C03 (48 mg, 0.35 mmol), 1,4-dioxane (3.5 mL) and H20 (0.5 mL), and then purged with nitrogen for three times. The nitrogen protected mixture was treated with Pd(dppf)Cl2 (13 mg, 0.017 mmol). The reaction mixture under nitrogen protection was then stirred at 90°C for 12 hrs. After being cooled to r.t., the mixture was diluted with EtOAc (50 mL), and washed with water and brine. The organic layer was separated, dried over MgSC^ and concentrated under reduced pressure. The residue was initially purified by Prep-TLC (EtOAc:MeOH=10: l) to get the crude product, which was purified by prep-HPLC to afford compound 711(6.1 mg, yield 6.2%).
1H NMR (400MHz, CD3OD) δ 7.65-7.77 (m, 8H), 7.33 (s, IH), 7.24 (s, IH), 5.18-5.20
(m, IH), 4.25 (d, J=7.2 Hz, IH), 3.86-4.02 (m, 2H), 3.88 (s, 3H), 3.67 (s, 3H), 2.01-2.38
(m, 5H), 0.91-1.02 (m, 6H). MS (ESI) m/z (M+H)+ 586.1.
[0203] The following compounds were prepared using General Procedure XI-
U:
-VIf
[0204] Compound 712; 2.5 mg, yields 3.3%. 1H NMR (300MHz, CD3OD) δ 7.62-7.77 (m, 8H), 7.30 (s, 1H), 7.20 (s, 1H), 5.13-5.19 (m, 1H), 4.21-4.28 (m, 3H), 3.83- 4.03 (m, 2H), 3.64 (s, 3H), 1.98-2.39 (m, 5H), 1.29-1.36 (m, 3H), 0.88-0.98 (m, 6H). MS (ESI) m/z (M+H)+ 600.3.
Scheme XI-VIg
[0205] Compound 713; 10 mg, yield 11%; 1H NMR (400MHz, CD3OD) δ
7.65-7.77 (m, 8H), 7.33 (s, 1H), 7.23 (s, 1H), 5.18-5.21 (m, 1H), 4.25 (d, J=7.2 Hz, 1H), 4.20 (t, J=7.2 Hz, 2H), 3.86-4.06 (m, 2H), 3.67 (s, 3H), 2.01-2.41 (m, 5H), 1.71-1.80 (m, 2H), 0.91-1.05 (m, 9H). MS (ESI) m/z (M+H)+ 614.1.
Example XI -VII: Preparation of Compounds 714-720
-Vla Xl-Vlla
Xl-Vla Xl-Vlla
General Procedure XI- V
[0206] To a solution compound XI-VIa (200 mg, 0.59 mmol) in 1,4-dioxane
(3 mL) was added isocyanate (1.3 mmol). The reaction mixture was heated to 80°C for 5 hrs. After being cooled to r.t., the mixture was diluted with EtOAc (40 mL), washed with water and brine. The organic layer was separated, dried over MgSC^, filtered and concentrated under reduced pressure. The residue was purified by Prep-TLC (Petroleum ether: EtOAc =3: 1) to afford general compound XI- Vila.
[0207] The following compounds were prepared using General Procedure XI-
Xl-Vllag
-VIIb
dioxane/H20, 90°C R=i-Pr, 716
R=Et, 717
R=Bu, 718
General Procedure XI-W
[0208] A flask was charged with general compound XI-VIIa (1 eq.), compound XI-IIc (1.05 eq.), CS2CO3 (2.2 eq.), 1,4-dioxane and H20 (0.05 mmol/mL, v/v=7/l), and then purged with nitrogen for three times. The nitrogen protected mixture was treated with Pd(dppf)Cl2 (10 mol%). The reaction mixture was stirred at 90°C for 12 hrs under nitrogen protection. After being cooled to r.t., the mixture was diluted with
EtOAc (50 mL), washed with water and brine. The organic layer was separated, dried over Na2S04, filtered and concentrated under reduced pressure to give a residue. Each residue was separately purified by Prep-TLC (Petroleum ether: EtOAc =1 :2) to afford each of the final compounds.
[0209] The following compounds were prepared using General Procedure XI-
W:
[0210] Compound 714; 8 mg, yield 6%. 1H NMR (400MHz, CD3OD): δ 7.59- 7.74 (m, 8H), 7.30 (s, 1H), 7.13 (s, 1H), 5.14-5.18 (m, 1H), 4.22 (d, J=7.5 Hz, 1H), 3.81-
4.04 (m, 2H), 3.64 (s, 3H), 3.22 (t, J=3.9 Hz, 2H), 1.92-2.40 (m, 5H), 1.51-1.64 (m, 2H), 0.88-1.00 (m, 9H). MS (ESI) m/z (M+H)+ 613.3.
[0211] Compound 715; 3.0 mg, yields 4%. 1H NMR (400MHz, CD3OD): δ
7.63-7.73 (m, 8H), 7.31 (s, 1H), 7.15 (s, 1H), 5.16-5.19 (m, 1H), 4.22-4.24 (m, 1H), 3.87- 4.03 (m, 2H), 3.65 (s, 3H), 2.02-2.37 (m, 5H), 1.41 (s, 9H), 0.89-0.95 (m, 6H). MS (ESI) m/z (M+H)+ 627.0.
[0212] Compound 716; 6.0 mg, yields 4%. 1H NMR (400MHz, CD3OD): δ 7.63-7.73 (m, 8H), 7.31 (s, 1H), 7.16 (s, 1H), 5.16-5.19 (m, 1H), 4.22-4.24 (m, 1H), 3.86- 4.01 (m, 3H), 3.65 (s, 3H), 2.02-2.37 (m, 5H), 1.23 (d, J=6.4 Hz, 6H), 0.89-0.95 (m, 6H). MS (ESI) m/z (M+H)+ 613.0.
H
[0213] Compound 717; 8.0 mg, yields 4%. 1H NMR (300MHz, CD3OD): δ 7.64-7.74 (m, 8H), 7.42 (s, 1H), 7.20 (s, 1H), 5.15-5.20 (m, 1H), 4.20-4.23 (m, 1H), 3.82- 4.05 (m, 2H), 3.64 (s, 3H), 1.99-2.41 (m, 7H), 1.17-1.22 (m, 3H), 0.88-0.94 (m, 6H). MS (ESI) m/z (M+H)+ 599.2.
[0214] Compound 718; 3.2 mg, yields 12%. XH NMR (300MHz, CD3OD): δ 7.60-7.81 (m, 8H), 7.33 (brs, IH), 7.17 (s, IH), 5.13-5.26 (m, IH), 4.23 (d, J=6.8Hz, IH), 3.87-4.08 (m, 2H), 3.65 (s, 3H), 3.23-3.29 (m, 2H), 1.96-2.39 (m, 5H), 1.51-1.63 (m, 2H), 1.37-1.50 (m, 2H), 0.88-1.03 (m, 9H). MS (ESI) m/z (M+H)+ 627.0.
[0215] Compound 719; 3.2 mg, yield 18%; 1H NMR (400MHz, CD3OD): δ
7.62-7.72 (m, 8H), 7.26-7.33 (m, 5H), 7.14-7.24 (m, 2H), 5.14-5.20 (m, IH), 4.23 (d,
J=7.2 Hz, IH), 3.84-4.05 (m, 2H), 3.65 (s, 3H), 3.45-3.57 (m, 2H), 2.84-2.90 (m, 2H),
1.97-2.41 (m, 5H), 0.89-1.00 (m, 6H). MS (ESI) m/z (M+H)+ 675.3.
[0216] Compound 720; 5 mg, yields 4%. 1H NMR (400MHz, CD3OD): δ
7.73-8.11 (m, 8H), 7.36-7.43 (m, 5H), 7.26-7.28 (m, IH), 7.10-7.20 (m, IH), 5.35-5.36
(m, IH), 4.24-4.26 (m, IH), 4.01-4.05 (m, IH), 3.91-3.99 (m, 1H),3.62 (s, 3H), 2.32-2.39
(m, 4H), 2.05-2.22 (m, 2H), 1.58-1.64 (m, 3H), 0.85-1.09 (m, 6H). MS (ESI) m/z
(M+H)+ 675.2.
Example XI -VIII: Preparation of Compounds 721 :
heme XI- VIII
General Procedure XI-X
[0217] A flask were charged with compound XI- Via (255 mg, 0.75 mmol),
N-Boc-L-phenylalanine (200 mg, 0.75 mmol), BOP (66 mg, 1.5 mmol), and DIEA (290 mg, 2.3 mmol) followed by addition of DMF (5 mL). The resulting mixture was stirred at
80°C overnight. After being cooled to r.t., the mixture was diluted with EtOAc (20 mL), washed with water and brine, dried over anhydrous Na2S04 and concentrated under reduced pressure. The residue was purified by prep-TLC (DCM/MeOH=20/l) to afford compound XI-VIIIa (60 mg, yield 16%). MS (ESI) m/z (M+H)+ 487.
-VIIIb
General Procedure XI-XI
[0218] A flask was charged with compound XI-VIIIa (40 mg, 0.08 mmol), compound XI-IIc (41 mg, 0.08 mmol), Cs2C03 (52 mg, 0.16 mmol), DME (3 mL) and
H20 (0.5 mL), and then purged with nitrogen for three times. The nitrogen protected mixture was treated with Pd(dppf)Cl2 (20 mg, 0.027 mmol). The resulting mixture was stirred at 80°C for 3 hrs under nitrogen protection. After being cooled to r.t., the mixture was diluted with EtOAc (50 mL), and washed with water and brine. The organic layer was separated, dried over Na2S04, filtered and concentrated under reduced pressure. The residue was purified by Prep-HPLC to afford compound 721 (4.5 mg, yield 7%). *H
NMR (400MHz, CD3OD): δ 7.82-7.90 (m, 9 H), 7.54 (s, 1H), 7.23-7.33 (m, 5 H), 5.23-
5.27 (m, 1 H), 4.50-4.54 (m, 1 H), 4.22-4.24 (m, 1 H), 4.09-4.14 (m, 1 H), 3.84-3.91 (m, 1
H), 3.66 (s, 3 H), 3.20-3.25 (m, 1 H), 2.96-3.01 (m, 1 H), 2.55-2.60 (m, 1 H), 2.03-2.30
(m, 4 H), 1.39 (s, 9 H), 0.89-0.94 (m, 6 H). MS (ESI) m/z (M+H)+ 775.1.
Example XII-IX: Preparation of Compound 808
Scheme XII-IX
Scheme X-I
General Procedure X-W
[0219] To a mixture of 2-methyl-L-proline (1.0 g, 7.8 mmol) in 20 mL of dry methanol was added SOCl2 (2.8 g, 23.3 mmol) dropwise at 0°C under nitrogen protection.
The resulting mixture was stirred at room temperature overnight, and then the solvent was removed under reduced pressure to afford compound I-IVa as an HC1 salt (1.4 g, yield
100%). 1H NMR (300 MHz, CD3OD): δ 3.86 (s, 3H), 3.42-3.46 (m, 2H), 2.36-2.45 (m,
1H), 2.00-2.19 (m, 3H), 1.68 (s, 3H).
Scheme X-IVb
General Procedure X-X
[0220] To a solution of compound I-IVa (1.35 g, 7.7 mmol) in 30 mL of
DCM was added compound Vl-IIa (1.5 g, 8.5 mmol), HATU (4.4 g, 11.6 mmol) and
DIEA (3 g, 23 mmol). The resulting mixture was stirred at room temperature overnight.
Subsequently, the mixture was diluted with DCM and washed with brine. The organic layers were dried over anhydrous Na2S04 and concentrated. The residue was purified by
column chromatography (PE/EA=3/1) to afford compound l-IVb (1.5 g, yield 65%). MS (ESI) m/z (M+H)+ 301.
Scheme X-IVc
General Procedure X-Y
[0221] A mixture of compound l-IVb (1.5 g, 5 mmol) and NaOH (0.6 g, 15 mmol) in MeOH (30 mL) and H20 (5 mL) was stirred at 70°C for 2 hours. The methanol under reduce pressure and the residue was dissolved with 20 mL of H20, then the solution was acidfied to pH 2-3 with 2N HC1 and extracted with DCM (50 mLx2). The organic layer was washed with brine, dried over anhydrous Na2S04 and concentrated to afford compound l-IVc (0.8 g, yield 57%), which was used in next step without further purification. 1H NMR (300 MHz, DMSO-d6): δ 12.20 (s, 1H), 7.24 (d, J=8.4 Hz, 1 H),
3.54-3.98 (m, 3H), 3.50 (s, 3H), 1.78-2.05 (m, 5H), 1.34 (s, 3H), 0.84-0.88 (m, 6H).
Scheme XII-IXa
General Procedure XII-AW
[0222] The mixture of compound XII-IIc (100 mg, 0.25 mmol), compound I-
IVc (145 mg, 0.50 mmol), Cs2C03 (244 mg, 0.75 mmol) in DMF (5 mL) was stirred at room temperature for 2 hrs. The mixture was diluted with EtOAc (50 mL), and washed with water and brine. The organic layer was separated, dried over anhydrous Na2S04, filtered and concentrated in vacuo to give a crude residue. The residue was purified by prep-TLC (DCM/MeOH=20/l) to afford compound XII-IXa (100 mg, yield 50%). MS
(ESI) m/z (M+H)+ 807.
Scheme XII-IXb
General Procedure XII-AX
[0223] A mixture of compound Xll-IXa (100 mg, 0.12 mmol) and NH4OAc
(185 mg, 2.4 mmol) in xylene (10 mL) was stirred at 120°C for 5 hours in a sealed tube.
After being cooled to r.t., the solvent was removed under reduce pressure and the resulting residue was diluted with EtOAc (30 mL), and washed with water and brine. The organic layer was separated, dried over Na2S04, filtered and concentrated under reduced pressure. The residue was purified by Prep-HPLC to afford compound 808 (12 mg, yield
14%). 1H NMR (400 MHz, CD3OD): δ 7.64-7.75 (m, 8H), 7.30 (s, 2H), 4.18-4.20 (m,
2H), 3.88-4.08 (m, 4H), 3.65 (s, 6H), 2.50-2.57 (m, 2H), 2.01-2.16 (m, 8H) ,1.86 (s, 6H),
0.87-0.98 (m, 12H). MS (ESI) m/z (M+H)+ 767.4.
General Procedure XII-AY
[0224] A flask was charged with compound XII-IVa (100 mg, 0.17 mmol), compound I-IVc (50 mg, 0.17 mmol), Cs2C03 (222 mg 0.68 mmol) and DMF (10 mL).
The resulting mixture was stirred at room temperature for 3 hours. Subsequently, water
(30 mL) was added, and the mixture was extracted with EtOAc (30 mL x 2). The combined organic layers were washed with brine, dried over Na2S04, and concentrated to afford compound XII-Xa (90 mg, yield 67%), which was used in next step without further purification.
Scheme XII-Xb
General Procedure XII-AZ
[0225] A mixture of compound XII-Xa (0.09 g, 0.11 mmol) and NH4OAc
(0.175 g, 2.27 mmol) in xylene (10 mL) was stirred at 120-130°C for 4 hrs in a sealed tube. After cooling to room temperature, water (30 mL) was added, and the mixture was extracted with EtOAc (30 mL x 2). The combined organic layers were washed with brine, dried over Na2S04 and concentrated to dryness to give a residue. The residue was purified by Prep-HPLC to afford compound 809 (6 mg, yield 10%). 1H NMR (400MHz,
CD3OD): δ 7.60-7.86 (m, 8 H), 7.20-7.25 (m, 2H), 5.14-5.20 (m, 1H), 4.14-4.20 (m, 2 H),
3.81-4.09 (m, 4 H), 3.62(s, 6 H), 2.49-2.51 (m, 1 H), 2.09-2.35 (m, 5 H), 1.94-2.04 (m, 4 H), 1.83 (s, 3 H), 0.83-0.96 (m, 12 H). MS (ESI) m / z (M+H)+ 753.5.
SECTION XIII PREPARATION OF COMPOUNDS: SECTION XIII
Example XIII-I: Synthesis of Intermediates for the Preparation of Compound 900
Scheme XIII-I
Xlll-la
Scheme XIII-Ia
General Procedure XIII-A
[0226] To a stirred and cooled (0°C, ice bath) solution of L-Valine methyl ester hydrochloride (10 g, 60 mmol) in water (60 mL) were added dropwise aq. Na2C03 (1 M solution, 66 mL) and methyl chloro formate (7 g, 72 mmol). The ice-bath was then removed and the mixture stirred at r.t. for 3 hours. Subsequently, the mixture was extracted with EtOAc (60 mL x 3), dried over MgS04 and concentrated to afford N- (methoxycarbonyl)-L-Valine methyl ester (10 g, yield 88%), used in next step without further purification. 1H NMR (400MHz, CDC13): δ 5.22 (br d, 1 H), 4.29-4.24 (m, 1 H), 3.73 (s, 3 H), 3.69 (s, 3H), 2.18-2.10 (m, 1 H), 0.94 (d, J=8 Hz, 3 H), 0.88 (d, J=8 Hz, 3 H).
Scheme XIII-Ib
Xlll-la
General Procedure XIII-B
[0227] A solution of N-(methoxycarbonyl)-L-V aline methyl ester (0.95 g, 5 mmol) in toluene (10 mL) was degassed with nitrogen. Then it was cooled to -78°C using a dry ice-acetone bath. A solution of DIBAL in toluene (1 M, 12.5 mL, 12.5 mmol) was added dropwise at -78°C. After addition, the mixture was stirred at -78°C for 2 hrs. Subsequently, methanol (5 mL) was added slowly to quench the reaction while maintaining the temperature below -65°C. The resulting mixture was poured into 50 mL of aq. NaOH (1 M), then extracted with EtOAc (50 mL x 3), the combined extracts were washed with brine, dried over Na2S04, filtered and concentrated under reduced pressure to afford aldehyde XIII-Ia. To prevent racemization, the crude product was used directly in next step without further purification (0.8 g, crude yield 100%). 1H NMR (400MHz, CDC13): δ 9.62 (s, 1 H), 5.38 (brs, 1 H), 4.28 (brm, 1 H), 3.71 (s, 3H), 2.28 (m, 1H), 1.02 (d, J=6.8 Hz, 3 H), 0.90 (d, J=6.8 Hz, 3 H).
Example XIII-II: Synthesis of Intermediates for the Preparation of Compound 900
-II
General Procedure XIII-C
[0228] To a solution of compound IX-IIIf (0.2 g, 0.4 mmol), compound I- XVIIaa (0.2 g, 0.4 mmol), K2C03 (0.13 g, 1.0 mmol) in dioxane/H20 (2 mL / 0.5 mL) was added Pd(dppf)Cl2 (20 mg). The mixture was stirred at reflux overnight. After cooling to room temperature, the mixture was diluted with H20 (20 mL), and extracted with EtOAc (30 mL x 3). The combined organic layers were washed with brine (10 mL), dried over anhydrous Na2S04, and concentrated in vacuo. The residue was purified by Prep-TLC to afford compound XHI-IIa (0.15 g, yield 50%). -IIb
General Procedure XIII-D
[0229] A solution of compound XHI-IIa (0.15 g, 0.3 mmol) in TFA/DCM (2 mL/2 mL) was stirred at room temperature for 1 hour. The mixture was concentrated under reduced pressure to afford crude product (0.16 g, crude yield 100%) of which 30 mg of the crude product was purified by Prep-HPLC to afford 15 mg of compound XIII- IIb. 1H NMR (400MHz, CD3OD): δ 7.57-7.79 (m, 8 H), 7.29-7.36 (m, 2 H), 5.15-5.18 (m, 1 H), 4.34-4.37 (m, 1 H), 4.21-4.25 (m, 1 H), 3.98-4.01 (m, 1 H), 3.78-3.87 (m, 1 H), 3.67 (s, 3 H), 3.00-3.18 (m, 2 H), 2.14-2.48 (m, 4 H), 1.80-2.11 (m, 5 H), 0.97-0.93 (m, 6 H). MS (ESI) m / z (M+H)+ 582.1.
Example XIII-III: Preparation of Compound 900
-III
General Procedure XIII-E
[0230] To a solution of compound XIII-IIb (0.1 g, 0.17 mmol) in CH3OH (5 mL), CH3COOH (0.5 mL) was added compound XIII-Ia (28 mg, 0.18 mmol) and stirred 30 min. Subsequently, NaCNBH3 (0.033 g, 0.52 mmol) was added to the stirring mixture. The mixture was stirred at room temperature for an additional 3 hours. The mixture was then concentrated, and the residue was diluted with H20, and extracted with DCM. The organic layer was separated, dried over Na2S04 and concentrated, the residue was purified by Prep-HPLC to afford compound 900 (25 mg, yield 21%). 1H NMR (400MHz, CD3OD): δ 7.63-7.79 (m, 8H), 7.37 (s, 1 H), 7.30 (s, 1 H), 5.15-5.18 (m, 1H), 4.22 (d, J=7.6 Hz, 1 H), 3.68-3.97 (m, 2 H), 3.62 (s, 3 H), 3.65 (s, 3 H), 3.49-3.55 (m, 2 H), 2.65-2.70 (m, 1 H), 1.87-2.39 (m, 13 H), 0.87-0.99 (m, 6 H), 0.67-0.86 (m, 6 H). MS (ESI) m / z (M+H)+ 725.4.
[0231] Example IX-IV: Compound 901 can be prepared according to the following Scheme:
[0232] Example IX-V: Preparation of compound 902:
Scheme XIII-V
General Procedure XIII-F
[0233] A solution of 4,4'-stilbenediamine (50 mg, 0.174 mmol) in anhydrous DCM (3 mL) was treated with compound I-IVc (115 mg, 0.4mmol), HATU (166 mg, 0.436 mmol) and DIEA (90 mg, 0.697 mmol). The resulting mixture was stirred at r.t. for 4 hours. The mixture was diluted with water (10 mL) and extracted with EtOAc (30 mL x 2). The combined organic layers were dried over sodium sulfate, and concentrated in vacuo to give a residue. The residue was purified by Prep-HPLC to afford compound 902 (30 mg, yield 23%). 1H NMR (400 MHz, CD3OD): δ 7.49-7.56 (m, 8H), 7.09 (s, 2H), 4.19 (d, J=7.6Hz, 2H), 3.98-3.99 (m, 2H), 3.83-3.89 (m, 2H), 3.68 (s, 6H), 2.34-2.41 (m, 2H), 2.08-2.16 (m, 6H), 1.91-2.00 (m, 2H), 1.69 (s, 6H), 1.03 (d, J=6.4Hz, 6H), 0.97 (d, J=6.4Hz, 6H). MS (ESI) m/z (M+H)+ 747.5.
[0234] Example IX- VI: Compound 903 can be prepared according to the following Scheme:
[0235] Example IX- VII: Compound 904 can be prepared according to the following Scheme:
[0236] Example IX- VIII: Compound 905 can be prepared according to the following Scheme:
-119-
[0238] Example IX-X: Compound 907 can be prepared according to the following Scheme:
[0239] Example IX-XI: Compound 908 can be prepared according to the following Scheme:
Pd(PPh3)4, Na2C03, Toluene-H20
Example IX-XII: Compound 909 can be prepared according to the following Scheme:
SECTION III
PREPARATION OF COMPOUNDS: SECTION III
Example XIV-I: Synthesis of Compound 950
XIV-la XIV-I b
XlV-ld -le
XlV-Ia XlV-Ib
General Procedure XIV -A
[0240] Compound XlV-Ia (80 mg, 0.2 mmol) was dissolved in a solution of TFA in DCM (30%, 2 mL). The resulting mixture was stirred at r.t. for 1 h. After concentration under reduced pressure, the residue was neutralized with aq. NaOH (1 M), and extracted with DCM (30 mL x 3). The combined organic layers were washed with brine, dried over Na2S04, filtered and concentrated to afford compound XlV-Ib (50 mg, yield 83.0%), which was used directly in the next step without further purification. MS (ESI) m/z (M+H)+ 306.
Scheme XlV-Ib
[0241] A mixture of compound XlV-Ib (50 mg, 0.16 mmol), compound VII- IIA (43 mg, 0.25 mmol), HATU (95 mg, 0.25 mmol) and DIEA (65 mg, 0.5 mmol) in DCM was stirred at r.t for 2 hrs. The mixture was diluted with DCM (60 mL), and washed with brine. The organic layer was separated, dried over Na2S04, filtered and concentrated. The residue was purified by prep-TLC (PE: EtOAc =1 :2) to afford compound XIV-Ic (50 mg, yield 66%). MS (ESI) m/z (M+H)+464. -Ic
XlV-ld XlV-le
General Procedure XIV -C
[0242] A flask was charged with compound XlV-ld (300 mg, 0.75 mmol), bis(pinacolato)diboron (380 mg, 1.5 mmol), K2CO3 (205 mg, 1.5 mmol), 1 ,4-dioxane (10 mL) and H20 (lmL), and then purged with nitrogen three times. Subsequently, the nitrogen protected mixture was treated with Pd(dppf)Cl2 (50 mg, 0.068 mmol). The resulting mixture was stirred at 80°C for 3 hrs under nitrogen protection. After cooling to r.t., the mixture was diluted with EtOAc (100 mL), and washed with water and brine. The organic layer was separated, dried over Na2S04, filtered and concentrated under reduced pressure to give a residue. The residue was purified by prep-TLC (PE/ EtOAc =2/1) to afford the compound X-Ie (150 mg, yield 45%). MS (ESI) m/z (M+H)+ 449. -
General Procedure XIV -D
[0243] A mixture of compound XlV-le (150 mg, 0.33 mmol) and 10% Pd/C (20 mg) in MeOH (20 mL) was hydrogenated under H2 (45 psi) at 50°C. After stirring for 8 hrs, the mixture was cooled to r.t. and the H2 was replaced with N2 and then the catalyst
was removed by filtration. The filtrate was removed to afford compound XlV-lf (70 mg, yield 67%), which was used in next step directly. MS (ESI) m/z (M+H)+ 315.
Scheme XlV-I
General Procedure X-E
[0244] A mixture of compound XlV-lf (70 mg, 0.22 mmol), compound VII- IIA (46.2 mg, 0.26 mmol), HATU (99 mg, 0.26 mmol) and DIEA (67 mg, 0.52 mmol) in DCM was stirred at r.t for 2 hrs. Subsequently, the mixture was diluted with DCM (60 mL), and washed with water and brine. The organic layer was separated, dried over Na2S04, filtered and concentrated under reduced pressure to give a residue. The residue was purified by prep-TLC (PE: EtOAc =1 :2) to afford compound XlV-lg (50 mg, yield 48%). MS (ESI) m/z (M+H)+ 472. -lf
General Procedure XIV -F
[0245] A flask was charged with compound XlV-lg (50 mg, 0.11 mmol), compound XIV-Ic (50 mg, 0.1 mmol), K2CO3 (30 mg, 0.22 mmol), 1,4-dioxane (2 mL) and H20 (0.5 mL), and then purged with nitrogen three times. Subsequently, the nitrogen protected mixture was treated with Pd(dppf)Cl2 (10 mg, 0.0137 mmol). The resulting mixture was stirred at 90°C for 3 hrs under nitrogen protection. After cooling to r.t., the mixture was diluted with EtOAc (50 mL), and washed with water and brine. The organic layer was separated, dried over Na2S04, filtered and concentrated under reduced pressure to give a residue. The residue was purified by prep-HPLC to afford compound 950 (8 mg, yield: 11%). 1H NMR (400MHz, CD3OD): δ 7.73-7.83 (m, 3H), 7.62-7.69 (m, 4H), 7.31
(s, 1H), 5.28-5.32 (m, 1H), 4.17-4.27 (m, 2H), 4.04-4.10 (m, 2H), 3.90-3.94 (m, 2H), 3.64
(s, 6H), 2.43-2.55 (m, 2H), 1.86-2.18 (m, 8H), 1.86 (s, 3H), 0.86-1.06 (m, 12H). MS (ESI) m/z (M+H)+728.5.
SECTION IV
PREPARATION OF COMPOUNDS: SECTION IV
Example XV-I: Compound 1001 can be prepared according to the following Schemes:
-Ia
heme XV-Iaa
Example XV-II: Compound 1002 can be prepared according to the following Scheme:
-IIa
Example XV-III: Compound 1003 can be prepared according to the following Schemes:
-IIIa
Scheme XV-IIIaa
Example XV-IV: Compound 1004 can be prepared according to the following Schemes:
Scheme XV-IVa
Example XV- V: Compound 1005 can be prepared according to the following Schemes:
- Va
Scheme XV-VIa
Example XV- VII: Compound 1007 can be prepared according to the following Schemes:
-VIIa
Example XV- VIII: Compound 1008 can be prepared according to the following Schemes:
Scheme XV- illa
-VIIIaa
SECTION V
[0246] HCV Replicon Assay
[0247] Huh7 cells containing HCV replicons with an integrated luciferase reporter gene are maintained at 37°C in 5% C02 in Dulbecco's modified Eagle's medium (DMEM; Mediatech, Herndon, VA) containing 10% heat-inactivated fetal bovine serum (FBS; Mediatech, Herndon, VA), 2 mM L-glutamine (Cambrex Bioscience, WalkersviUe, MD), 1% non essential amino acids (Lonza, WalkersviUe, MD), 50 IU/mL penicillin
(Mediatech, Herndon, VA), 50 mg/mL streptomycin (Mediatech, Herndon, VA) and 0.5 mg/mL G418 (Promega, Madison, WI) . Cells are sub-divided 1 :3 or 4 every 2-3 days.
[0248] 24h prior to the assay, Huh7 cells containing sub-genomic HCV replicons are collected, counted, and plated in Nunclon 96-well tissue culture plates (ThermoFisher, Rochester, NY) at 5000 cells/well in lOOmL standard maintenance medium (above) and incubated in the conditions above. To initiate the assay, culture medium is removed, and replaced with 90 mL maintenance media lacking G418. Test compounds are serially diluted three-fold in dimethyl sulfoxide (DMSO) in two duplicate rows for each EC50 determination. These compound solutions are diluted ten-fold in DMEM lacking serum and G418. 10 mL of these compound solutions in media are added to duplicate tissue culture plates. The final volume is 100 with a DMSO concentration of 1%. Compound concentrations are adjusted to appropriately define a dose response curve. Typical dilution series range from lOOmM to 1.69 nM final concentrations to InM to 16.9 fM final. Plates are incubated at 37 °C for approximately 48 hr.
[0249] Following incubation, media is removed from one of the two duplicate plates and replicon-reporter luciferase activity is measured using a Bright-Glo luciferase assay kit (Promega, Madison, WI) according to manufacturer's instructions. Semi-log plots of luciferase activity versus the logarithm of compound concentration are fit to a 4- parameter logistic function using XLfit software (IDBS Inc., Guildford, UK) to determine
[0250] Table 1 : Examples of activity.
716 C
717 B
718 B
719 B
720 C
721 C
808 C
809 C
900 B
902 A
904 C
905 C
906 A
907 B
908 C
909 C
1001 C
[0251] A indicates an EC50 of 100 nM and greater than 100 nM
[0252] B indicates an EC50 between 10 and 100 nM
[0253] C indicates an EC50 of 10 nM and less than 10 nM
Claims
WHAT IS CLAIMED IS:
1. A compound having the structure of Formula XV:
□ 12 13
R ^N/ R
I
L6— L4
L7-Q7
XV
or a pharmaceutically acceptable salt thereof,
wherein:
12 13 12 13
each R R TSi is separately selected, wherein R and R are each separately selected from hydrogen, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab. or R12R13N is a heterocyclyl linked through a ring nitrogen atom optionally substituted with one or more of oxo, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl,
heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3-7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab. each Rlab is separately selected from the group consisting of -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80,
Ci_6alkylC(=0)Ci_6alkyl, aryl, aryl(CH2)„-, aryl(CH2)nO-, aryl(CH=CH)m-, arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)„-, (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R80 is separately selected from the group consisting of hydrogen, alkoxyalkyl, Ci_6alkyl, C3_7cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and (ReRfN)alkyl, said alkoxyalkyl, Ci_6alkyl, C3_7cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (ReRfN)alkyl are each optionally substituted with one or more Rlac;
each Rlac is separately selected from the group consisting of -C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-,
Ci_6alkylC(=0)Ci_6alkyl, aryl, aryl(CH2)„-, aryl(CH2)nO-, aryl(CH=CH)m-
arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)n-, (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each Y14 is separately selected from the group consisting of -C(=0)-, - S(=0)-, -C(=S)-, -S(=0)2-, -C(=0)0-, -C(=0)NR2\ -S(=0)2NR2\ - C(=0)NR2cC(=0)-, and -C(CF3)2NR2c-,
R12 R13
N
sisting
Y2 is selected from O (oxygen), S (sulfur), S(O), S02, NR2, and C(R2)2 with the proviso that when X2 is null Y2 is C(R2)2;
each X10 is (C(R2)2)q;
each Y11 is separately selected from the group consisting of -0(C(R2)2)n-, 2)2)n-, -S(0)(C(R2)2)n-, -S02(C(R2)2)n-, -NR2(C(R2)2)n-, and (C(R2)2)q; R1 is selected from the group consisting of Rlaa, RlaC(=0)- and
RlaC(=S)-;
each Rla is separately selected from the group consisting of -C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-, Ci_6alkylOC(=0)Ci_6alkyl,
arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)n-, (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
Rlaa is -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s- (Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab.
each R10 is RcRdN-;
each R11 is separately selected from the group consisting of H (hydrogen), alkoxyalkyl,
aryl(CH2)n- arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN(CH2)„-, (RcRdN)alkyl, and Ci_6alkyl optionally substituted with up to 9 halo;
each RcRdN is separately selected, wherein Rc and Rd are each separately selected from hydrogen, alkoxyC(=0)-, Ci_6alkyl, Ci_6alkylC(=0)-,
Ci_6alkylsulfonyl, arylalkylOC(=0)-, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-, wherein the alkyl part of arylalkyl, arylalkylC(=0)-, heterocyclylalkyl, and heterocyclylalkylC(=0)- are each optionally substituted with one ReRfN- group; and wherein the aryl part of arylalkyl, arylalkylC(=0)-, arylC(=0)-, and arylsulfonyl, and the heterocyclyl part of heterocyclylalkyl, heterocyclylalkylC(=0)-, and heterocyclylC(=0)- are each optionally substituted with up to three substituents each independently selected from the group consisting of cyano, halo, nitro, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each ReRfN is separately selected, wherein Re and Rf are each separately selected from hydrogen, Ci_6alkyl, aryl, arylalkyl, cycloalkyl, (cyclolalkyl)alkyl, heterocyclyl, heterocyclylalkyl, (RxRyN)alkyl, and (RxRyN)C(=0)-;
each RxRyN is separately selected, wherein Rx and Ry are each separately selected from hydrogen, Ci_6alkylOC(=0)-, Ci_6alkyl, Ci_6alkylC(=0)-, aryl, arylalkyl, cycloalkyl, and heterocyclyl;
each C(R2a)2 is separately selected, wherein each R2a is separately selected from the group consisting of hydrogen, Ci_6alkyl optionally substituted with up to 9 halo, aryl(CH2)n-, and heteroaryl(CH2)n-, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo and Ci_6alkyl optionally substituted with up to 9
each R3a is separately selected from the group consisting of hydrogen, and optionally substituted Ci_6alkyl;
each R3b is separately selected from the group consisting of optionally substituted Ci_6alkyl, heteroaryl, -(CH2)nC(=0)NR4aR4b, -(CH2)nC(=0)OR5a, and -(CH2)nC(=0)R6a said heteroaryl optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R4aR4bN is separately selected, wherein R4a and R4b are each separately selected from the group consisting of hydrogen, optionally substituted Ci_6alkyl, and aryl(CH2)n-;
each R5a is separately selected from the group consisting of optionally substituted Ci_6alkyl, and aryl(CH2)„-;
each R6a is separately selected from the group consisting of optionally substituted Ci_6alkyl, and aryl(CH2)„-;
each A1 is separately selected from the group consisting of C2_6alkenyl, Ci_6alkyl, and -(CH2)n-0-(CH2)m-, each optionally substituted with one or more
R2;
each R2 is separately selected, wherein R2 is selected from the group consisting of hydrogen, halo, hydroxy, Ci_6alkoxy, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, alkyoxyalkyl, C3_7Cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, heterocyclylalkyl, (ReRfN)alkyl, and RaRbN-, said Ci_6alkyl optionally substituted with one or more halo, -OR2b, -C(=0)OR2b, - C(=0)NHR2b, -NHC (=NH)NHR2b , -NHR2b, SR2b, imidazolyl, indolyl, -SCH3, phenyl, and 4-hydroxyphenyl, said Ci_6alkoxy, alkoxyalkyl, aryl, C2_6alkenyl, C2_6alkynyl, C3_7Cycloalkyl, arylalkyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (ReRfN)alkyl each optionally substituted with one or more R4, or optionally two vicinal R2 and the carbons to which they are attached are together a fused three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups;
R2b is selected from the group consisting of hydrogen, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl;
each R2c is selected from the group consisting of hydrogen, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl, said alkyl optionally substituted with ReRfN-, alkoxy, or Ci_6alkylS-;
each RaRbN is separately selected, wherein Ra and Rb are each separately selected from the group consisting of hydrogen, C2_6alkenyl, and Ci_6alkyl;
L4 is selected from the group consisting of -(J2)S-(L5)S-(J2)S-(L5)S-J2-
, -C(=0)-, O (oxygen), - o
OC(R2)2-, ¾ H s -C(CF3)2NR2c-, NH, and -(CH=CH)-;
J2 is aryl, heteroaryl, heterocyclyl, cycloalkyl, cycloalkenyl, or polycyclic hydrocarbon, each optionally substituted with one or more R15;
each R14 is separately selected from the group consisting of hydroxy, Ci_6alkoxy optionally substituted with up to 9 fluoro, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R15 is separately selected from the group consisting of halo, hydroxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, R9, RxRyN, RxRyNC(=0), RxRyNCi_6alkyl, heteroaryl, aryl, Ci_6alkyl optionally substituted with up to 9 halo, Ci_6alkyl substituted with up to 5 hydroxy, Ci_6alkoxy optionally substituted with up to 9 halo, d_6haloalkyl, RaRbN-, (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl substituted with up to 5 hydroxy, said substituent aryl and heteroaryl are each optionally substituted with one or more R14, or optionally two vicinal R15 and the carbons to which they are attached are together a fused three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups, or optionally two geminal R15 and the carbon to which they are attached are together a three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups, or optionally two geminal R15 are together oxo;
C(R2)2, -C(R2)20-, -C(=0)-, O (oxygen), NH, and -(CH=CH)-;
each A is separately selected from the group consisting of CR3 and N (nitrogen);
each R is separately selected from the group consisting of hydrogen, Ci_6alkoxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, hydroxy, RaRbN- (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy;
L6 is selected from the group consisting of
se ecte rom t e group cons st ng o
each X4 is separately selected from the group consisting of CR4 and N
(nitrogen);
each Y4 is separately selected from the group consisting of C(R4)2, NR4, O (oxygen), and S (sulfur);
each X9 is separately selected from the group consisting of CH and N (nitrogen);
each Y10 is separately selected from the group consisting of -CH2- and -
NH-;
each m separately is 1 or 2;
each n separately is 0, 1 or 2;
each p separately is 1, 2, 3 or 4;
each q separately is 1, 2, 3, 4 or 5;
each r separately is 0, 1, 2, 3, or 4;
each s separately is 0 or 1 ;
each R4 is separately selected from the group consisting of H (hydrogen), Ci_6alkoxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, Ci_6haloalkyl, hydroxy, RaRbN-, (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy, or optionally two geminal R4 are together oxo;
R9 is selected from the group consisting of hydrogen and -C(=0)R9a; R9a is selected from the group consisting of -NR9bR9c, -OR9d, Ci_ Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
R9b is selected from the group consisting of hydrogen, Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
R9c is selected from the group consisting of Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl; and
R9d is selected from the group consisting of Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl.
2. The compound of Claim 1, wherein at least one R12R13N is a nitrogen containing heterocyclyl optionally including one or more -C(=0)-, O (oxygen), S (sulfur), S(O), S02, or NH groups in the heterocyclyl ring and optionally substituted with one or more of -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl,
C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more R1.
optionally substituted with one or more of -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-
[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-
Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3_7CycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3-7cycloalkyl,
Ci_6alkylC(=0)-, C3-7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more R1; wherein Y3 is O (oxygen), S (sulfur), S(O), S02, -C(=0)-, -NH-, -CH2-,
-NHCH2-, -OCH2-, -SCH2-, -NHCH2-, -NHC(=0)-, or -NHS02-.
4. The compound of Claim 1 having the Formula XVa:
5. The compound of Claim 1, having the structure:
-147-
or a pharmaceutically acceptable salt thereof.
6. The compound of Claim 1, wherein L4 is selected from the group
each B is separately selected, wherein B is a fused optionally substituted saturated or unsaturated three- to seven-membered carbocyclic ring or a fused optionally substituted saturated or unsaturated three- to seven-membered heterocyclic ring, each optionally substituted with one or more R4;
L2 is selected from the group consisting of -C(=0)-, -(CH2CH2)-, -(CH20)-, -(CH2S)-, -(CH=CH)-, -(CH=N)-, -NH-, O (oxygen), S (sulfur), and -CH2-;
L3 is selected from the group consisting of
■(NR )-, O
(oxygen), S (sulfur), and -CH2-;
X3 is selected from the group consisting of NH, O (oxygen), and S (sulfur);
each Xs is separately selected from the group consisting of -NH-, O (oxygen), S (sulfur), and -CH2-;
each X6 is separately selected from the group consisting of N (nitrogen), and CR ; and
each R8 is separately selected from the group consisting of hydrogen, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, halo, (RaRbN)alkyl, (RaRbN)C(=0)-, and Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy.
e group
R6 is Ci_6alkyl optionally substituted with up to 9 halo.
The compound of Claim 8, wherein R6 is methyl.
A compound havin the structure of Formula XVI:
or a pharmaceutically acceptable salt thereof,
wherein:
each Y2 is selected from O (oxygen), S (sulfur), S(O), S02, NR2, and C(R2)2 with the proviso that when X2 is null Y2 is C(R2)2;
each X10 is (C(R2)2)q;
each Y11 is separately selected from the group consisting of -0(C(R2)2)n-, -S(C(R2)2)„-, -S(0)(C(R2)2)n-, -S02(C(R2)2)n-, -NR2(C(R2)2)„-, and (C(R2)2)q;
12 13 12 13
each R R TSi is separately selected, wherein R and R are each separately selected from hydrogen, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl,
C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl,
heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab. or R12R13N is a heterocyclyl linked through a ring nitrogen atom optionally substituted with one or more of oxo, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab.
each Rlab is separately selected from the group consisting of -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80,
arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)n- (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted
with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R80 is separately selected from the group consisting of hydrogen, alkoxyalkyl, Ci_6alkyl, C3-7cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and (ReRfN)alkyl, said alkoxyalkyl, Ci_6alkyl, C3_7Cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (ReRfN)alkyl are each optionally substituted with one or more Rlac;
each Rlac is separately selected from the group consisting of -C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-, Ci_6alkylOC(=0)Ci_6alkyl, Ci_6alkylC(=0)Ci_6alkyl, aryl, aryl(CH2)„- aryl(CH2)nO-, aryl(CH=CH)m- arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)„-, (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each Y14 is separately selected from the group consisting of -C(=0)-, - S(=0)-, -C(=S)-, -S(=0)2-, -C(=0)0-, -C(=0)NR2\ -S(=0)2NR2\ - C(=0)NR2cC(=0)-, and -C(CF3)2NR2c-,
R1 is selected from the group consisting of Rlaa, RlaC(=0)- and RlaC(=S)-;
each Rla is separately selected from the group consisting of -C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-, Ci_6alkylOC(=0)Ci_6alkyl, Ci_6alkylC(=0)Ci_6alkyl, aryl, aryl(CH2)„-, aryl(CH2)nO-, aryl(CH=CH)m-, arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)„-, (RcRdN)(CH=CH)m- (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted
with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
Rlaa is selected from the group consisting of -C(R2a)2NR3aR3b, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab.
each R10 is RcRdN-;
each R11 is separately selected from the group consisting of H (hydrogen), alkoxyalkyl,
aryl(CH2)n-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN(CH2)„-, (RcRdN)alkyl, and Ci_6alkyl optionally substituted with up to 9 halo;
each RcRdN is separately selected, wherein Rc and Rd are each separately selected from hydrogen, alkoxyC(=0)-, Ci_6alkyl, Ci_6alkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-, wherein the alkyl part of arylalkyl, arylalkylC(=0)-, heterocyclylalkyl, and heterocyclylalkylC(=0)- are each optionally substituted with one ReRfN- group; and wherein the aryl part of arylalkyl, arylalkylC(=0)-, arylC(=0)-, and arylsulfonyl, and the heterocyclyl
part of heterocyclylalkyl, heterocyclylalkylC(=0)-, and heterocyclylC(=0)- are each optionally substituted with up to three substituents each independently selected from the group consisting of cyano, halo, nitro, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each ReRfN is separately selected, wherein Re and Rf are each separately selected from hydrogen, Ci_6alkyl, aryl, arylalkyl, cycloalkyl, (cyclolalkyl)alkyl, heterocyclyl, heterocyclylalkyl, (RxRyN)alkyl, and (RxRyN)C(=0)-;
each RxRyN is separately selected, wherein Rx and Ry are each separately selected from hydrogen, Ci_6alkylOC(=0)-, Ci_6alkyl, Ci_6alkylC(=0)-, aryl, arylalkyl, cycloalkyl, and heterocyclyl;
each C(R2a)2 is separately selected, wherein each R2a is separately selected from the group consisting of hydrogen, Ci_6alkyl optionally substituted with up to 9 halo, aryl(CH2)„-, and heteroaryl(CH2)„-, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo and Ci_6alkyl optionally substituted with up to 9
each R3a is separately selected from the group consisting of hydrogen, and optionally substituted Ci_6alkyl;
each R3b is separately selected from the group consisting of optionally substituted d_6alkyl, heteroaryl, -(CH2)nC(=0)NR4aR4b, -(CH2)nC(=0)OR5a, and -(CH2)nC(=0)R6a said heteroaryl optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R4aR4bN is separately selected, wherein R4a and R4b are each separately selected from the group consisting of hydrogen, optionally substituted Ci_6alkyl, and aryl(CH2)„-;
each R5a is separately selected from the group consisting of optionally substituted Ci_6alkyl, and aryl(CH2)n-;
each R6a is separately selected from the group consisting of optionally substituted Ci_6alkyl, and aryl(CH2)„-;
each A1 is separately selected from the group consisting of C2_6alkenyl, Ci_6alkyl, and -(CH2)n-0-(CH2)m-, each optionally substituted with one or more
R2;
each R2 is separately selected, wherein R2 is selected from the group consisting of hydrogen, halo, hydroxy, Ci_6alkoxy, Ci_6alkyl, C2-6alkenyl, C2_6alkynyl, alkyoxyalkyl, C3_7Cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, heterocyclylalkyl, (ReRfN)alkyl, RaRbN-, said Ci_6alkyl optionally substituted with one or more halo, -OR2b, -C(=0)OR2b, - C(=0)NHR2b, -NHC (=NH)NHR2b , -NHR2b, SR2b, imidazolyl, indolyl, -SCH3, phenyl, and 4-hydroxyphenyl, said Ci_6alkoxy, alkoxyalkyl, aryl, C2_6alkenyl, C2_6alkynyl, C3_7Cycloalkyl, arylalkyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (ReRfN)alkyl each optionally substituted with one or more R4, or optionally two vicinal R2 and the carbons to which they are attached are together a fused three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups;
R2b is selected from the group consisting of hydrogen, Ci_6alkyl, C2_6alkenyl, C2-6alkynyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl;
each R2c is selected from the group consisting of hydrogen, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl, said alkyl optionally substituted with ReRfN-, alkoxy, or Ci_6alkylS-;
each RaRbN is separately selected, wherein Ra and Rb are each separately selected from the group consisting of hydrogen, C2_6alkenyl, and Ci_6alkyl;
4 is selected from the group consisting of -(J2)s-(L5)s-(J2)s-(L5)s-J2-i
J2 is aryl, heteroaryl, heterocyclyl, cycloalkyl, cycloalkenyl, or polycyclic hydrocarbon, each optionally substituted with one or more R15;
each R14 is separately selected from the group consisting of hydroxy, Ci_6alkoxy optionally substituted with up to 9 fluoro, Ci_6alkylOCi_6alkyl,
Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R15 is separately selected from the group consisting of halo, hydroxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, R9, RxRyN, RxRyNC(=0), RxRyNCi_6alkyl, heteroaryl, aryl, Ci_6alkyl optionally substituted with up to 9 halo, Ci_6alkyl substituted with up to 5 hydroxy, Ci_6alkoxy optionally substituted with up to 9 halo, Ci_6haloalkyl, RaRbN-, (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl substituted with up to 5 hydroxy, said substituent aryl and heteroaryl are each optionally substituted with one or more R14, or optionally two vicinal R15 and the carbons to which they are attached are together a fused three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups, or optionally two geminal R15 and the carbon to which they are attached are together a three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups, or optionally two geminal R15 are together oxo; 5 is separately selected from the group consisting of
Ci_6alkoxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, hydroxy, RaRbN- (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy;
L6 is selected from the group consisting of
each X4 is separately selected from the group consisting of CR4 and N
(nitrogen);
each Y4 is separately selected from the group consisting of C(R4)2, NR4, O (oxygen), and S (sulfur);
each X9 is separately selected from the group consisting of CH and N (nitrogen);
each Y10 is separately selected from the group consisting of -CH2- and -
NH-;
each m separately is 1 or 2;
each n separately is 0, 1 or 2;
each p separately is 1, 2, 3 or 4;
each q separately is 1, 2, 3, 4 or 5;
each r separately is 0, 1, 2, 3, or 4;
each s separately is 0 or 1 ;
each R4 is separately selected from the group consisting of H (hydrogen), Ci_6alkoxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, Ci_6haloalkyl, hydroxy, RaRbN-, (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy, or optionally two geminal R4 are together oxo;
R9 is selected from the group consisting of hydrogen and -C(=0)R9a;
R9a is selected from the group consisting of -NR9bR9c, -OR9d, Ci_ Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
R9b is selected from the group consisting of hydrogen, Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
R9c is selected from the group consisting of Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl; and
R9d is selected from the group consisting of Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl.
11. The compound of Claim 10, having the structure:
12. The compound of Claim 10, wherein R6 is methyl.
13. The compound of Claim 10 havin the structure of Formula XVIa:
XVIa
or a pharmaceutically acceptable salt thereof, wherein R6 is Ci_6alkyl optionally substituted with up to 9 halo.
14. The compound of Claim 10, wherein
is selected from the group consisting of -C(=0)-, -(CH2CH2)-, -(CH2S)-, -(CH=CH)-, -(CH=N)-, -NH-, O (oxygen), S (sulfur), and o
L3 is selected from the group consisting of H , -(NR9)-, O (oxygen), S (sulfur), and -CH2-;
each X3 is separately selected from the group consisting of NH, O (oxygen), and S (sulfur);
each Xs is separately selected from the group consisting of -NH-, O (oxygen), S (sulfur), and -CH2-;
each X6 is separately selected from the group consisting of N (nitrogen), and CR8; and
each B is separately selected, wherein B is a fused optionally substituted saturated or unsaturated three- to seven-membered carbocyclic ring or a fused optionally substituted saturated or unsaturated three- to seven-membered heterocyclic ring, each optionally substituted with one or more R4
XVII
or a pharmaceutically acceptable salt thereof,
wherein:
□ 12 p 13 selected from the group consisting of ■n~v , and
each Y2 is selected from O (oxygen), S (sulfur), S(O), S02, NR2, and C(R2)2 with the proviso that when X2 is null Y2 is C(R2)2;
each X10 is (C(R2)2)q;
each Y11 is separately selected from the group consisting of -0(C(R2)2)n-,
-S(C(R2)2)n-, -S(0)(C(R2)2)n-, -S02(C(R2)2)n-, -NR2(C(R2)2)n-, and (C(R2)2)q;
12 13 12 13
each R R TSi is separately selected, wherein R and R are each separately selected from hydrogen, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl,
heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3-7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab.
or R12R13N is a heterocyclyl linked through a ring nitrogen atom optionally substituted with one or more of oxo, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab.
each Rlab is separately selected from the group consisting of -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80,
arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)n-, (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R80 is separately selected from the group consisting of hydrogen, alkoxyalkyl, Ci_6alkyl, C3-7cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and (ReRfN)alkyl, said alkoxyalkyl, Ci_6alkyl, C3_7Cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (ReRfN)alkyl are each optionally substituted with one or more Rlac;
each Rlac is separately selected from the group consisting of -C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-, Ci_6alkylOC(=0)Ci_6alkyl,
arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)n-, (RcRdN)(CH=CH)m- (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each Y14 is separately selected from the group consisting of -C(=0)-, - S(=0)-, -C(=S)-, -S(=0)2-, -C(=0)0-, -C(=0)NR2\ -S(=0)2NR2\ - C(=0)NR2cC(=0)-, and -C(CF3)2NR2c-,
R1 is selected from the group consisting of Rlaa, RlaC(=0)- and RlaC(=S)-;
each Rla is separately selected from the group consisting of -C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-, Ci_6alkylOC(=0)Ci_6alkyl,
arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)n-, (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
Rlaa is selected from the group consisting of -C(R2a)2NR3aR3b, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3-7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab.
each R10 is RcRdN-;
each R11 is separately selected from the group consisting of H (hydrogen), alkoxyalkyl, Ci_6alkylOC(=0)Ci_6alkyl, Ci_6alkylC(=0)Ci_6alkyl, aryl(CH2)„-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN(CH2)n-, (RcRdN)alkyl, and Ci_6alkyl optionally substituted with up to 9 halo;
each RcRdN is separately selected, wherein Rc and Rd are each separately selected from hydrogen, alkoxyC(=0)-, Ci_6alkyl, Ci_6alkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-, wherein the alkyl part of arylalkyl, arylalkylC(=0)-, heterocyclylalkyl, and heterocyclylalkylC(=0)- are each optionally substituted with one ReRfN- group; and wherein the aryl part of arylalkyl, arylalkylC(=0)-, arylC(=0)-, and arylsulfonyl, and the heterocyclyl part of heterocyclylalkyl, heterocyclylalkylC(=0)-, and heterocyclylC(=0)- are each optionally substituted with up to three substituents each independently selected from the group consisting of cyano, halo, nitro, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each ReRfN is separately selected, wherein Re and Rf are each separately selected from hydrogen, Ci_6alkyl, aryl, arylalkyl, cycloalkyl, (cyclolalkyl)alkyl, heterocyclyl, heterocyclylalkyl, (RxRyN)alkyl, and (RxRyN)C(=0)-;
each RxRyN is separately selected, wherein Rx and Ry are each separately selected from hydrogen, Ci_6alkylOC(=0)-, Ci_6alkyl, Ci_6alkylC(=0)-, aryl, arylalkyl, cycloalkyl, and heterocyclyl;
each C(R2a)2 is separately selected, wherein each R2a is separately selected from the group consisting of hydrogen, Ci_6alkyl optionally substituted with up to 9 halo, aryl(CH2)„-, and heteroaryl(CH2)„-, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo and Ci_6alkyl optionally substituted with up to 9
each R3a is separately selected from the group consisting of hydrogen, and optionally substituted Ci_6alkyl;
each R3b is separately selected from the group consisting of optionally substituted Ci_6alkyl, heteroaryl, -(CH2)nC(=0)NR4aR4b, -(CH2)nC(=0)OR5a, and -(CH2)nC(=0)R6a said heteroaryl optionally substituted with cyano, halo,
nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R4aR4bN is separately selected, wherein R4a and R4b are each separately selected from the group consisting of hydrogen, optionally substituted Ci_6alkyl, and aryl(CH2)n-;
each R5a is separately selected from the group consisting of optionally substituted Ci_6alkyl, and aryl(CH2)„-;
each R6a is separately selected from the group consisting of optionally substituted Ci_6alkyl, and aryl(CH2)„-;
each A1 is separately selected from the group consisting of C2_6alkenyl, Ci_6alkyl, and -(CH2)n-0-(CH2)m-, each optionally substituted with one or more
R2;
each R2 is separately selected, wherein R2 is selected from the group consisting of protium, halo, hydroxy, Ci_6alkoxy, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, alkyoxyalkyl, C3_7Cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, heterocyclylalkyl, (ReRfN)alkyl, RaRbN-, said Ci_6alkyl optionally substituted with one or more halo, -OR2b, -C(=0)OR2b, - C(=0)NHR2b, -NHC (=NH)NHR2b , -NHR2b, SR2b, imidazolyl, indolyl, -SCH3, phenyl, and 4-hydroxyphenyl, said Ci_6alkoxy, alkoxyalkyl, aryl, C2_6alkenyl, C2_6alkynyl, C3_7Cycloalkyl, arylalkyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (ReRfN)alkyl each optionally substituted with one or more R4, or optionally two vicinal R2 and the carbons to which they are attached are together a fused three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups;
R2b is selected from the group consisting of hydrogen, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl;
each R2c is selected from the group consisting of hydrogen, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl, said alkyl optionally substituted with ReRfN-, alkoxy, or Ci_6alkylS-;
each RaRbN is separately selected, wherein Ra and Rb are each separately selected from the group consisting of hydrogen, C2_6alkenyl, and Ci_6alkyl;
4 is selected from the group consisting of -J 2 -(L 5 )S-(J 2 )S-(L 5 )S-J 2 - -J 2 -
, -C(=0)-, O (oxygen), - o
OC(R2)2-, ¾ H f -C(CF3)2NR2c-, NH, and -(CH=CH)-;
each J2 is phenyl optionally substituted with one or more R15;
each R14 is separately selected from the group consisting of hydroxy, Ci_6alkoxy optionally substituted with up to 9 fluoro, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R15 is separately selected from the group consisting of halo, hydroxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, R9, RxRyN, RxRyNC(=0), RxRyNCi_6alkyl, heteroaryl, aryl, d_6alkyl optionally substituted with up to 9 halo, Ci_6alkyl substituted with up to 5 hydroxy, Ci_6alkoxy optionally substituted with up to 9 halo, Ci_6haloalkyl, RaRbN-, (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl substituted with up to 5 hydroxy, said substituent aryl and heteroaryl are each optionally substituted with one or more R14, or optionally two vicinal R15 and the carbons to which they are attached are together a fused three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups, or optionally two geminal R15 and the carbon to which they are attached are together a three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups, or optionally two geminal R15 are together oxo; each L5 is separately selected from the group consisting of
,
^ = = ^T^^ , -C(CF3)2NR2c- -C(R2)2- -C(=0)-, O (oxygen), NH, and -(CH=CH)-;
each A is separately selected from the group consisting of CR3 and N (nitrogen);
L6 is selected from the group consisting of
7 is selected from the group consisting of
C(R2)20-, -C(=0)-, O (oxygen), NH, and -(CH=CH)-;
each X4 is separately selected from the group consisting of CR4 and N (nitrogen);
each Y4 is separately selected from the group consisting of C(R4)2, NR4, O (oxygen), and S (sulfur);
each X is separately selected from the group consisting of CH and N (nitrogen);
each Y10 is separately selected from the group consisting of -CH2- and -
each m separately is 1 or 2;
each n separately is 0, 1 or 2;
each p separately is 1, 2, 3 or 4;
each q separately is 1, 2, 3, 4 or 5;
each r separately is 0, 1, 2, 3, or 4;
each s separately is 0 or 1 ;
each R3 is separately selected from the group consisting of hydrogen, Ci_6alkoxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, hydroxy, RaRbN- (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy;
each R4 is separately selected from the group consisting of H (hydrogen),
Ci_6alkoxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, Ci_6haloalkyl, hydroxy, RaRbN-, (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy, or optionally two geminal R4 are together oxo;
R6 is Ci_6alkyl optionally substituted with up to 9 halo;
R is selected from the group consisting of hydrogen and -C(=0)R a;
R9a is selected from the group consisting of -NR9bR9c, -OR9d, Ci_ Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
R9b is selected from the group consisting of hydrogen, Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
R9c is selected from the group consisting of Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl; and
R9d is selected from the group consisting of Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl.
16. The compound of Claim 15, wherein
or a pharmaceutically acceptable salt thereof.
18. A compound having the structure of Formula XVIII :
Q16
I
[_16 |_14
L I 17_L18
I
Q17
XVIII
, and r2 y
R1^ /R13 selected from the group consisting
R1^ / R 13 of Q16 and 17 is not ■™w
each Y2 is selected from O (oxygen), S (sulfur), S(O), S02, NR , and C(R2)2 with the proviso that when X2 is null Y2 is C(R2)2;
each X10 is (C(R2)2)q;
each Y11 is separately selected from the group consisting of -0(C(R2)2)n-, -S(C(R2)2)n-, -S(0)(C(R2)2)n-, -S02(C(R2)2)n-, -NR2(C(R2)2)n-, and (C(R2)2)q;
12 13 12 13
each R R TSi is separately selected, wherein R and R are each separately selected from hydrogen, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl,
C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3-7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab.
or R12R13N is a heterocyclyl linked through a ring nitrogen atom optionally substituted with one or more of oxo, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
each R a is separately selected from the group consisting of -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-[Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]-Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80,
arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)n-, (RcRdN)(CH=CH)m- (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R80 is separately selected from the group consisting of hydrogen, alkoxyalkyl, Ci_6alkyl, C3-7cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and (ReRfN)alkyl, said alkoxyalkyl, Ci_6alkyl, C3_7Cycloalkyl, aryl, arylalkyl, heterocyclyl, heterocyclylalkyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (ReRfN)alkyl are each optionally substituted with one or more Rlac;
each Rlac is separately selected from the group consisting of -C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-, Ci_6alkylOC(=0)Ci_6alkyl,
arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)n- (RcRdN)(CH=CH)m- (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each Y14 is separately selected from the group consisting of -C(=0)-, - S(=0)-, -C(=S)-, -S(=0)2-, -C(=0)0-, -C(=0)NR2\ -S(=0)2NR2\ - C(=0)NR2cC(=0)-, and -C(CF3)2NR2c-,
each R1 is separately selected from the group consisting of Rlaa, RlaC(=0)- and RlaC(=S)-;
each Rla is separately selected from the group consisting of -C(R2a)2NR3aR3b, alkoxyalkyl, Ci_6alkylOC(=0)-, Ci_6alkylOC(=0)Ci_6alkyl,
arylalkylO-, arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)(CH=CH)m-, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclyl, heterocyclyl(CH=CH)m-, heterocyclylalkoxy, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN- , RcRdN(CH2)n- (RcRdN)(CH=CH)m-, (RcRdN)alkyl, (RcRdN)C(=0)-, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each Rlaa is separately selected from the group consisting of -C(R2a)2NR3aR3b, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- [Y14(C(R2)2)r(NR2)s(C(R2)2)r]s-(Y14)s-R80, -[(Y14)(C(R2)2)r(NR2)s(C(R2)2)r]- Y14(C(R2)2)rO(C(R2)2)r-(Y14)s-R80, alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3-7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-,
said alkoxyalkyl, alkoxyC(=0)-, Ci_6alkyl, C3_7cycloalkyl, Ci_6alkylC(=0)-, C3_7cycloalkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, aryl, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, heteroarylC(=0)-, heteroarylalkylC(=0)-, and alkyl in (ReRfN)alkyl and (ReRfN)alkylC(=0)- are each optionally substituted with one or more
Rlab.
each R10 is RcRdN-;
each R is separately selected from the group consisting of H (hydrogen), alkoxyalkyl, Ci_6alkylOC(=0)Ci_6alkyl, Ci_6alkylC(=0)Ci_6alkyl, aryl(CH2)„- arylalkyl, arylOalkyl, cycloalkyl, (cycloalkyl)alkyl, cycloalkylOalkyl, heterocyclylalkyl, heterocyclylOalkyl, hydroxyalkyl, RcRdN(CH2)n-, (RcRdN)alkyl, and Ci_6alkyl optionally substituted with up to 9 halo;
each RcRdN is separately selected, wherein Rc and Rd are each separately selected from hydrogen, alkoxyC(=0)-, Ci_6alkyl, Ci_6alkylC(=0)-, Ci_6alkylsulfonyl, arylalkylOC(=0)-, arylalkyl, arylalkylC(=0)-, arylC(=0)-, arylsulfonyl, heterocyclylalkyl, heterocyclylalkylC(=0)-, heterocyclylC(=0)-, (ReRfN)alkyl, (ReRfN)alkylC(=0)-, and (ReRfN)C(=0)-, wherein the alkyl part of arylalkyl, arylalkylC(=0)-, heterocyclylalkyl, and heterocyclylalkylC(=0)- are each optionally substituted with one ReRfN- group; and wherein the aryl part of arylalkyl, arylalkylC(=0)-, arylC(=0)-, and arylsulfonyl, and the heterocyclyl part of heterocyclylalkyl, heterocyclylalkylC(=0)-, and heterocyclylC(=0)- are each optionally substituted with up to three substituents each independently selected from the group consisting of cyano, halo, nitro, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each ReRfN is separately selected, wherein Re and Rf are each separately selected from hydrogen, Ci_6alkyl, aryl, arylalkyl, cycloalkyl, (cyclolalkyl)alkyl, heterocyclyl, heterocyclylalkyl, (RxRyN)alkyl, and (RxRyN)C(=0)-;
each RxRyN is separately selected, wherein Rx and Ry are each separately selected from hydrogen, Ci_6alkylOC(=0)-, Ci_6alkyl, Ci_6alkylC(=0)-, aryl, arylalkyl, cycloalkyl, and heterocyclyl;
each C(R2a)2 is separately selected, wherein each R2a is separately selected from the group consisting of hydrogen, Ci_6alkyl optionally substituted with up to 9 halo, aryl(CH2)n-, and heteroaryl(CH2)n-, said aryl and heteroaryl each optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo and Ci_6alkyl optionally substituted with up to 9
halo, or optionally C(R2a)2 is
each R3a is separately selected from the group consisting of hydrogen, and optionally substituted Ci_6alkyl;
each R3b is separately selected from the group consisting of optionally substituted Ci_6alkyl, heteroaryl, -(CH2)nC(=0)NR4aR4b, -(CH2)nC(=0)OR5a, and -(CH2)nC(=0)R6a said heteroaryl optionally substituted with cyano, halo, nitro, hydroxyl, Ci_6alkoxy optionally substituted with up to 9 halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R4aR4bN is separately selected, wherein R4a and R4b are each separately selected from the group consisting of hydrogen, optionally substituted Ci_6alkyl, and aryl(CH2)„-;
each R5a is separately selected from the group consisting of optionally substituted Ci_6alkyl, and aryl(CH2)„-;
each R6a is separately selected from the group consisting of optionally substituted Ci_6alkyl, and aryl(CH2)„-;
each A1 is separately selected from the group consisting of C2_6alkenyl, Ci_6alkyl, and -(CH2)n-0-(CH2)m-, each optionally substituted with one or more
R2;
each R2 is separately selected, wherein R2 is selected from the group consisting of hydrogen, halo, hydroxy, Ci_6alkoxy, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, alkyoxyalkyl, C3_7Cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, heterocyclylalkyl, (ReRfN)alkyl, RaRbN-, said Ci_6alkyl optionally substituted with one or more halo, -OR2b, -C(=0)OR2b, - C(=0)NHR2b, -NHC (=NH)NHR2b , -NHR2b, SR2b, imidazolyl, indolyl, -SCH3, phenyl, and 4-hydroxyphenyl, said Ci_6alkoxy, alkoxyalkyl, aryl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, arylalkyl, heterocyclyl, heteroaryl, heteroarylalkyl, heterocyclylalkyl, and alkyl in (ReRfN)alkyl each optionally substituted with one or more R4, or optionally two vicinal R2 and the carbons to which they are attached are together a fused three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups;
R2b is selected from the group consisting of hydrogen, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl;
each R c is selected from the group consisting of hydrogen, Ci_6alkyl, C2_6alkenyl, C2_6alkynyl, C3_7cycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, heterocyclyl, and heterocyclylalkyl, said alkyl optionally substituted with ReRfN-, alkoxy, or Ci_6alkylS-;
each RaRbN is separately selected, wherein Ra and Rb are each separately selected from the group consisting of hydrogen, C2_6alkenyl, and Ci_6alkyl;
L14 is -Jn-J2-J12-;
J2 is aryl, heteroaryl, heterocyclyl, cycloalkyl, cycloalkenyl, or polycyclic hydrocarbon, each optionally substituted with one or more R15;
J11 is C(R4)2, NR4, O (oxygen), or S (sulfur);
J12 is -C(=0)-, S(O), S02, or -C(=NR4)-;
each R14 is separately selected from the group consisting of hydroxy, Ci_6alkoxy optionally substituted with up to 9 fluoro, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, and Ci_6alkyl optionally substituted with up to 9 halo;
each R15 is separately selected from the group consisting of halo, hydroxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, R9, RxRyN, RxRyNC(=0), RxRyNCi_6alkyl, heteroaryl, aryl, Ci_6alkyl optionally substituted with up to 9 halo, Ci_6alkyl substituted with up to 5 hydroxy, Ci_6alkoxy optionally substituted with up to 9 halo, Ci_6haloalkyl, RaRbN-, (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl substituted with up to 5 hydroxy, said substituent aryl and heteroaryl are each optionally substituted with one or more R14, or optionally two vicinal R15 and the carbons to which they are attached are together a fused three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups, or optionally two geminal R15 and the carbon to which they are attached are together a three- to six-membered carbocyclic ring optionally substituted with up to two Ci_6alkyl groups, or optionally two geminal R15 are together oxo;
C(R2)2, -C(R2)20-, -C(=0)-, O (oxygen), NH, and -(CH=CH)-;
each A is separately selected from the group consisting of CR3 and N (nitrogen);
each R3 is separately selected from the group consisting of hydrogen, Ci_6alkoxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, hydroxy, RaRbN- (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy;
L17 is selected from the group consisting of aryl, heteroaryl, heterocyclyl, cycloalkyl, cycloalkenyl, or polycyclic hydrocarbon, each optionally substituted with one or more R15;
L18 is selected from the group consisting of
eac X s separate y se ected from the group consisting of CR and N (nitrogen);
each Y4 is separately selected from the group consisting of C(R4)2, NR4, O (oxygen), and S (sulfur);
each X9 is separately selected from the group consisting of CH and N (nitrogen);
each Y10 is separately selected from the group consisting of -CH2- and -
NH-:
each m separately is 1 or 2;
each n separately is 0, 1 or 2;
each p separately is 1, 2, 3 or 4;
each q separately is 1, 2, 3, 4 or 5;
each r separately is 0, 1, 2, 3, or 4;
each s separately is 0 or 1 ;
each R4 is separately selected from the group consisting of H (hydrogen), Ci_6alkoxy, Ci_6alkylOCi_6alkyl, Ci_6alkylOC(=0)-, arylalkylOC(=0)-, -COOH, halo, Ci_6haloalkyl, hydroxy, RaRbN-, (RaRbN)alkyl, (RaRbN)C(=0)-, Ci_6alkyl optionally substituted with up to 9 halo and up to 5 hydroxy, or optionally two geminal R4 are together oxo;
R9 is selected from the group consisting of hydrogen and -C(=0)R9a;
R9a is selected from the group consisting of -NR9bR9c, -OR9d, Ci_ Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
R9b is selected from the group consisting of hydrogen, Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl;
R9c is selected from the group consisting of Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl; and
R9d is selected from the group consisting of Ci_6alkyl optionally substituted with up to 9 halo, and optionally substituted aryl.
XVIIIa
or a pharmaceutically acceptable salt thereof,
B is a heterocyclyl, cycloalkyl, or polycyclic hydrocarbon, each optionally substituted with one or more R15; and
each R16 is separately hydrogen or R15.
The compound of Claim 19, wherein
14
B is aryl, or heteroaryl;
J11 is NR4; and
J12 is -C(=0)-.
21. The compound of Claim 20, wherein B10 is phenyl.
22. The com ound of Claim 20, wherein
each X is separately selected from the group consisting of CR and N
(nitrogen)
Y is selected from the group consisting of NR , O (oxygen), and S (sulfur)
each R17 is separately hydrogen or R15.
The compound of Claim 22, wherein
The compound of Claim 18 having the structure
-184-
or a pharmaceutically acceptable salt thereof.
A compound having the structure:
or a pharmaceutically acceptable salt thereof.
27. A pharmaceutical composition comprising a pharmaceutically acceptable excipient and a compound of any of the preceding claims.
28. A method of treating HCV infection in an individual, the method comprising administering to the individual an effective amount of a compound of any of Claims 1-26 or with the composition of Claim 27.
29. The method of Claim 28, further comprising identifying a subject suffering from a hepatitis C infection.
30. A method of treating liver fibrosis in an individual, the method comprising administering to the individual an effective amount of a compound of any of Claims 1-26 or with the composition of Claim 27.
31. The method of Claim 30, further comprising identifying a subject suffering from a hepatitis C infection.
32. A method of increasing liver function in an individual having a hepatitis C virus infection, the method comprising administering to the individual an effective amount of a compound of any of Claims 1-26 or with the composition of Claim 27.
33. The method of Claim 32, further comprising identifying a subject suffering from a hepatitis C infection.
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201061425718P | 2010-12-21 | 2010-12-21 | |
| US61/425,718 | 2010-12-21 | ||
| US201161454438P | 2011-03-18 | 2011-03-18 | |
| US61/454,438 | 2011-03-18 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2012087976A2 true WO2012087976A2 (en) | 2012-06-28 |
| WO2012087976A3 WO2012087976A3 (en) | 2012-11-29 |
Family
ID=46314813
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2011/065925 WO2012087976A2 (en) | 2010-12-21 | 2011-12-19 | Novel inhibitors of hepatitis c virus replication |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2012087976A2 (en) |
Cited By (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012068234A3 (en) * | 2010-11-17 | 2013-01-17 | 12Gilead Sciences, Inc. | Antiviral compounds |
| US8575135B2 (en) | 2011-11-16 | 2013-11-05 | Gilead Sciences, Inc. | Antiviral compounds |
| US8669234B2 (en) | 2009-05-13 | 2014-03-11 | Gilead Sciences, Inc. | Antiviral compounds |
| US8686026B2 (en) | 2010-06-10 | 2014-04-01 | Abbvie Inc. | Solid compositions |
| US8691938B2 (en) | 2009-06-11 | 2014-04-08 | Abbvie Inc. | Anti-viral compounds |
| US8937150B2 (en) | 2009-06-11 | 2015-01-20 | Abbvie Inc. | Anti-viral compounds |
| US9034832B2 (en) | 2011-12-29 | 2015-05-19 | Abbvie Inc. | Solid compositions |
| US9187496B2 (en) | 2009-12-18 | 2015-11-17 | Idenix Pharmaceuticals Llc | 5,5-fused arylene or heteroarylene hepatitis C virus inhibitors |
| CN105461701A (en) * | 2015-12-14 | 2016-04-06 | 上海步越化工科技有限公司 | Novel method for synthesizing anti-hepatitis C virus novel medicine daclatasvir |
| US9326973B2 (en) | 2012-01-13 | 2016-05-03 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US9333204B2 (en) | 2014-01-03 | 2016-05-10 | Abbvie Inc. | Solid antiviral dosage forms |
| US9340520B2 (en) | 2011-02-07 | 2016-05-17 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| CN105753944A (en) * | 2014-12-19 | 2016-07-13 | 正大天晴药业集团股份有限公司 | Preparation intermediate for daclatasvir and derivatives thereof |
| CN105753844A (en) * | 2016-02-16 | 2016-07-13 | 江苏苏利精细化工股份有限公司 | Novel method for synthesizing dimethyl dicarbamate dihydrochloride compound |
| CN105777719A (en) * | 2016-03-29 | 2016-07-20 | 安徽联创生物医药股份有限公司 | Synthesis method of Daclatasvir |
| CN106032388A (en) * | 2015-03-11 | 2016-10-19 | 浙江九洲药业股份有限公司 | Preparation method of Daclatasvir compound |
| CN106256825A (en) * | 2016-07-04 | 2016-12-28 | 四川同晟生物医药有限公司 | The synthetic method of his Wei of Dacca |
| US9546160B2 (en) | 2011-05-12 | 2017-01-17 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US9717712B2 (en) | 2013-07-02 | 2017-08-01 | Bristol-Myers Squibb Company | Combinations comprising tricyclohexadecahexaene derivatives for use in the treatment of hepatitis C virus |
| US9770439B2 (en) | 2013-07-02 | 2017-09-26 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US9775831B2 (en) | 2013-07-17 | 2017-10-03 | Bristol-Myers Squibb Company | Combinations comprising biphenyl derivatives for use in the treatment of HCV |
| CN107235884A (en) * | 2016-03-29 | 2017-10-10 | 上海医药工业研究院 | His Wei intermediate of a kind of Dacca and preparation method thereof |
| US10039779B2 (en) | 2013-01-31 | 2018-08-07 | Gilead Pharmasset Llc | Combination formulation of two antiviral compounds |
| US10086011B2 (en) | 2013-08-27 | 2018-10-02 | Gilead Pharmasset Llc | Combination formulation of two antiviral compounds |
| CN108640847A (en) * | 2018-06-25 | 2018-10-12 | 青岛大学 | Adjacent aminobenzophenone class compound of the one kind containing fluorene group |
| US10201541B1 (en) | 2011-05-17 | 2019-02-12 | Abbvie Inc. | Compositions and methods for treating HCV |
| US10456414B2 (en) | 2011-09-16 | 2019-10-29 | Gilead Pharmasset Llc | Methods for treating HCV |
| US10617675B2 (en) | 2015-08-06 | 2020-04-14 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US10800789B2 (en) | 2012-05-16 | 2020-10-13 | Gilead Pharmasset Llc | Antiviral compounds |
| US11191747B2 (en) | 2019-04-03 | 2021-12-07 | Aligos Therapeutics, Inc. | Pyrrole compounds |
| US11203599B2 (en) | 2014-06-11 | 2021-12-21 | Gilead Pharmasset Llc | Solid forms of an antiviral compound |
| US11484534B2 (en) | 2013-03-14 | 2022-11-01 | Abbvie Inc. | Methods for treating HCV |
| US11891382B2 (en) | 2017-04-26 | 2024-02-06 | Basilea Pharmaceutica International AG | Processes for the preparation of furazanobenzimidazoles and crystalline forms thereof |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107235965A (en) * | 2016-03-29 | 2017-10-10 | 上海医药工业研究院 | A kind of preparation method of his Wei of Dacca |
Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008021927A2 (en) * | 2006-08-11 | 2008-02-21 | Bristol-Myers Squibb Company | Hepatitis c virus inhibitors |
| WO2009102318A1 (en) * | 2008-02-12 | 2009-08-20 | Bristol-Myers Squibb Company | Hepatitis c virus inhibitors |
| WO2010065681A1 (en) * | 2008-12-03 | 2010-06-10 | Presidio Pharmaceuticals, Inc. | Inhibitors of hcv ns5a |
| WO2010091413A1 (en) * | 2009-02-09 | 2010-08-12 | Enanta Pharmaceuticals, Inc. | Linked dibenzimidazole derivatives |
| WO2010096462A1 (en) * | 2009-02-17 | 2010-08-26 | Enanta Pharmaceuticals, Inc | Linked diimidazole derivatives |
| WO2010099527A1 (en) * | 2009-02-27 | 2010-09-02 | Enanta Pharmaceuticals, Inc. | Hepatitis c virus inhibitors |
| WO2010111483A1 (en) * | 2009-03-27 | 2010-09-30 | Merck Sharp & Dohme Corp. | Inhibitors of hepatitis c virus replication |
| WO2010132601A1 (en) * | 2009-05-13 | 2010-11-18 | Gilead Sciences, Inc. | Antiviral compounds |
| US20100316607A1 (en) * | 2009-06-16 | 2010-12-16 | Yat Sun Or | Hepatitis c virus inhibitors |
| WO2011009084A2 (en) * | 2009-07-16 | 2011-01-20 | Vertex Pharmaceuticals Incorporated | Benzimidazole analogues for the treatment or prevention of flavivirus infections |
| WO2011031934A1 (en) * | 2009-09-11 | 2011-03-17 | Enanta Pharmaceuticals, Inc. | Hepatitis c virus inhibitors |
| WO2011060000A1 (en) * | 2009-11-12 | 2011-05-19 | Bristol-Myers Squibb Company | Hepatitis c virus inhibitors |
| WO2011149856A1 (en) * | 2010-05-24 | 2011-12-01 | Presidio Pharmaceuticals, Inc. | Inhibitors of hcv ns5a |
| WO2011150243A1 (en) * | 2010-05-28 | 2011-12-01 | Presidio Pharmaceuticals, Inc. | Inhibitors of hcv ns5a |
-
2011
- 2011-12-19 WO PCT/US2011/065925 patent/WO2012087976A2/en active Application Filing
Patent Citations (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008021927A2 (en) * | 2006-08-11 | 2008-02-21 | Bristol-Myers Squibb Company | Hepatitis c virus inhibitors |
| WO2009102318A1 (en) * | 2008-02-12 | 2009-08-20 | Bristol-Myers Squibb Company | Hepatitis c virus inhibitors |
| WO2010065681A1 (en) * | 2008-12-03 | 2010-06-10 | Presidio Pharmaceuticals, Inc. | Inhibitors of hcv ns5a |
| WO2010065668A1 (en) * | 2008-12-03 | 2010-06-10 | Presidio Pharmaceuticals, Inc. | Inhibitors of hcv ns5a |
| WO2010091413A1 (en) * | 2009-02-09 | 2010-08-12 | Enanta Pharmaceuticals, Inc. | Linked dibenzimidazole derivatives |
| WO2010096462A1 (en) * | 2009-02-17 | 2010-08-26 | Enanta Pharmaceuticals, Inc | Linked diimidazole derivatives |
| WO2010099527A1 (en) * | 2009-02-27 | 2010-09-02 | Enanta Pharmaceuticals, Inc. | Hepatitis c virus inhibitors |
| WO2010111483A1 (en) * | 2009-03-27 | 2010-09-30 | Merck Sharp & Dohme Corp. | Inhibitors of hepatitis c virus replication |
| WO2010132601A1 (en) * | 2009-05-13 | 2010-11-18 | Gilead Sciences, Inc. | Antiviral compounds |
| US20100316607A1 (en) * | 2009-06-16 | 2010-12-16 | Yat Sun Or | Hepatitis c virus inhibitors |
| WO2011009084A2 (en) * | 2009-07-16 | 2011-01-20 | Vertex Pharmaceuticals Incorporated | Benzimidazole analogues for the treatment or prevention of flavivirus infections |
| WO2011031934A1 (en) * | 2009-09-11 | 2011-03-17 | Enanta Pharmaceuticals, Inc. | Hepatitis c virus inhibitors |
| WO2011060000A1 (en) * | 2009-11-12 | 2011-05-19 | Bristol-Myers Squibb Company | Hepatitis c virus inhibitors |
| WO2011149856A1 (en) * | 2010-05-24 | 2011-12-01 | Presidio Pharmaceuticals, Inc. | Inhibitors of hcv ns5a |
| WO2011150243A1 (en) * | 2010-05-28 | 2011-12-01 | Presidio Pharmaceuticals, Inc. | Inhibitors of hcv ns5a |
Cited By (58)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8822430B2 (en) | 2009-05-13 | 2014-09-02 | Gilead Pharmasset Llc | Antiviral compounds |
| US9511056B2 (en) | 2009-05-13 | 2016-12-06 | Gilead Pharmasset Llc | Antiviral compounds |
| US8669234B2 (en) | 2009-05-13 | 2014-03-11 | Gilead Sciences, Inc. | Antiviral compounds |
| US9981955B2 (en) | 2009-05-13 | 2018-05-29 | Gilead Pharmasset Llc | Antiviral compounds |
| US8841278B2 (en) | 2009-05-13 | 2014-09-23 | Gilead Pharmasset Llc | Antiviral compounds |
| US8921514B2 (en) | 2009-06-11 | 2014-12-30 | Abbvie Inc. | Anti-viral compounds |
| US8691938B2 (en) | 2009-06-11 | 2014-04-08 | Abbvie Inc. | Anti-viral compounds |
| US10039754B2 (en) | 2009-06-11 | 2018-08-07 | Abbvie Inc. | Anti-viral compounds |
| US9586978B2 (en) | 2009-06-11 | 2017-03-07 | Abbvie Inc. | Anti-viral compounds |
| US8937150B2 (en) | 2009-06-11 | 2015-01-20 | Abbvie Inc. | Anti-viral compounds |
| US10028937B2 (en) | 2009-06-11 | 2018-07-24 | Abbvie Inc. | Anti-viral compounds |
| US9187496B2 (en) | 2009-12-18 | 2015-11-17 | Idenix Pharmaceuticals Llc | 5,5-fused arylene or heteroarylene hepatitis C virus inhibitors |
| US8686026B2 (en) | 2010-06-10 | 2014-04-01 | Abbvie Inc. | Solid compositions |
| EP3284741A1 (en) * | 2010-11-17 | 2018-02-21 | Gilead Pharmasset LLC | Antiviral compounds |
| AU2011328980B2 (en) * | 2010-11-17 | 2015-07-30 | Gilead Pharmasset Llc | Antiviral compounds |
| US9156823B2 (en) | 2010-11-17 | 2015-10-13 | Gilead Pharmasset Llc | Antiviral compounds |
| WO2012068234A3 (en) * | 2010-11-17 | 2013-01-17 | 12Gilead Sciences, Inc. | Antiviral compounds |
| US10344019B2 (en) | 2010-11-17 | 2019-07-09 | Gilead Pharmasset Llc | Antiviral compounds |
| US9340520B2 (en) | 2011-02-07 | 2016-05-17 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US9546160B2 (en) | 2011-05-12 | 2017-01-17 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US10201541B1 (en) | 2011-05-17 | 2019-02-12 | Abbvie Inc. | Compositions and methods for treating HCV |
| US10201584B1 (en) | 2011-05-17 | 2019-02-12 | Abbvie Inc. | Compositions and methods for treating HCV |
| US10456414B2 (en) | 2011-09-16 | 2019-10-29 | Gilead Pharmasset Llc | Methods for treating HCV |
| US8940718B2 (en) | 2011-11-16 | 2015-01-27 | Gilead Pharmasset Llc | Antiviral compounds |
| US10807990B2 (en) | 2011-11-16 | 2020-10-20 | Gilead Pharmasset Llc | Antiviral compounds |
| US9221833B2 (en) | 2011-11-16 | 2015-12-29 | Gilead Pharmasset Llc | Antiviral compounds |
| US9051340B2 (en) | 2011-11-16 | 2015-06-09 | Gilead Pharmasset Llc | Antiviral compounds |
| US8921341B2 (en) | 2011-11-16 | 2014-12-30 | Gilead Pharmasset Llc | Antiviral compounds |
| US8575135B2 (en) | 2011-11-16 | 2013-11-05 | Gilead Sciences, Inc. | Antiviral compounds |
| US9868745B2 (en) | 2011-11-16 | 2018-01-16 | Gilead Pharmasset Llc | Antiviral compounds |
| US9809600B2 (en) | 2011-11-16 | 2017-11-07 | Gilead Pharmasset Llc | Antiviral compounds |
| US9034832B2 (en) | 2011-12-29 | 2015-05-19 | Abbvie Inc. | Solid compositions |
| US9326973B2 (en) | 2012-01-13 | 2016-05-03 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US10800789B2 (en) | 2012-05-16 | 2020-10-13 | Gilead Pharmasset Llc | Antiviral compounds |
| US10039779B2 (en) | 2013-01-31 | 2018-08-07 | Gilead Pharmasset Llc | Combination formulation of two antiviral compounds |
| US11484534B2 (en) | 2013-03-14 | 2022-11-01 | Abbvie Inc. | Methods for treating HCV |
| US9770439B2 (en) | 2013-07-02 | 2017-09-26 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| US9717712B2 (en) | 2013-07-02 | 2017-08-01 | Bristol-Myers Squibb Company | Combinations comprising tricyclohexadecahexaene derivatives for use in the treatment of hepatitis C virus |
| US9775831B2 (en) | 2013-07-17 | 2017-10-03 | Bristol-Myers Squibb Company | Combinations comprising biphenyl derivatives for use in the treatment of HCV |
| US11116783B2 (en) | 2013-08-27 | 2021-09-14 | Gilead Pharmasset Llc | Combination formulation of two antiviral compounds |
| US11707479B2 (en) | 2013-08-27 | 2023-07-25 | Gilead Sciences, Inc. | Combination formulation of two antiviral compounds |
| US10086011B2 (en) | 2013-08-27 | 2018-10-02 | Gilead Pharmasset Llc | Combination formulation of two antiviral compounds |
| US9333204B2 (en) | 2014-01-03 | 2016-05-10 | Abbvie Inc. | Solid antiviral dosage forms |
| US10105365B2 (en) | 2014-01-03 | 2018-10-23 | Abbvie Inc. | Solid antiviral dosage forms |
| US9744170B2 (en) | 2014-01-03 | 2017-08-29 | Abbvie Inc. | Solid antiviral dosage forms |
| US11203599B2 (en) | 2014-06-11 | 2021-12-21 | Gilead Pharmasset Llc | Solid forms of an antiviral compound |
| CN105753944A (en) * | 2014-12-19 | 2016-07-13 | 正大天晴药业集团股份有限公司 | Preparation intermediate for daclatasvir and derivatives thereof |
| CN106032388A (en) * | 2015-03-11 | 2016-10-19 | 浙江九洲药业股份有限公司 | Preparation method of Daclatasvir compound |
| US10617675B2 (en) | 2015-08-06 | 2020-04-14 | Bristol-Myers Squibb Company | Hepatitis C virus inhibitors |
| CN105461701A (en) * | 2015-12-14 | 2016-04-06 | 上海步越化工科技有限公司 | Novel method for synthesizing anti-hepatitis C virus novel medicine daclatasvir |
| CN105753844A (en) * | 2016-02-16 | 2016-07-13 | 江苏苏利精细化工股份有限公司 | Novel method for synthesizing dimethyl dicarbamate dihydrochloride compound |
| CN105777719A (en) * | 2016-03-29 | 2016-07-20 | 安徽联创生物医药股份有限公司 | Synthesis method of Daclatasvir |
| CN107235884A (en) * | 2016-03-29 | 2017-10-10 | 上海医药工业研究院 | His Wei intermediate of a kind of Dacca and preparation method thereof |
| CN106256825A (en) * | 2016-07-04 | 2016-12-28 | 四川同晟生物医药有限公司 | The synthetic method of his Wei of Dacca |
| US11891382B2 (en) | 2017-04-26 | 2024-02-06 | Basilea Pharmaceutica International AG | Processes for the preparation of furazanobenzimidazoles and crystalline forms thereof |
| CN108640847A (en) * | 2018-06-25 | 2018-10-12 | 青岛大学 | Adjacent aminobenzophenone class compound of the one kind containing fluorene group |
| US11191747B2 (en) | 2019-04-03 | 2021-12-07 | Aligos Therapeutics, Inc. | Pyrrole compounds |
| US11771680B2 (en) | 2019-04-03 | 2023-10-03 | Aligos Therapeutics, Inc. | Pyrrole compounds |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2012087976A3 (en) | 2012-11-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2012087976A2 (en) | Novel inhibitors of hepatitis c virus replication | |
| US20110312996A1 (en) | Novel inhibitors of hepatitis c virus replication | |
| EP1999129B1 (en) | Compounds and methods for inhibiting hepatitis c viral replication | |
| US7932277B2 (en) | Peptide inhibitors of hepatitis C virus replication | |
| US7781474B2 (en) | Inhibitors of hepatitis C virus replication | |
| EP2177523A1 (en) | Novel macrocyclic inhibitors of hepatitis c virus replication | |
| US20110082182A1 (en) | Therapeutic antiviral peptides | |
| WO2012054874A1 (en) | Novel macrocyclic inhibitors of hepatitis c virus replication | |
| US20110129444A1 (en) | Novel macrocyclic inhibitors of hepatitis c virus replication | |
| WO2011075607A1 (en) | Novel inhibitors of hepatitis c virus replication | |
| WO2012047764A1 (en) | Therapeutic antiviral peptides | |
| WO2010045266A1 (en) | Therapeutic antiviral peptides | |
| WO2012037259A1 (en) | Novel inhibitors of hepatitis c virus replication | |
| US20110110890A1 (en) | Novel Inhibitors of Hepatitis C Virus Replication | |
| US20120251493A1 (en) | Novel macrocyclic inhibitors of hepatitis c virus replication |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11850070 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase in: |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11850070 Country of ref document: EP Kind code of ref document: A2 |